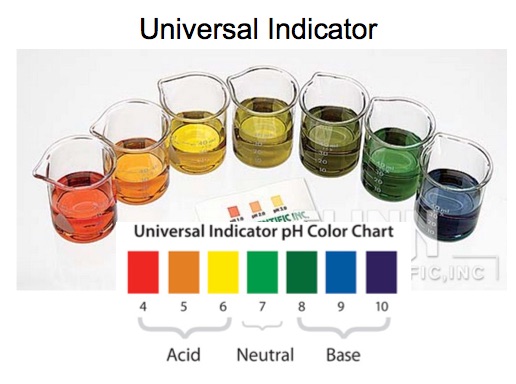
This demonstration focuses on imparting the concept of a buffer solution to students. Solutions having a pH of 4, 5, 6, 7, 8, 9, 10 are placed in separate labeled Erlenmeyer flasks. A few drops of Universal Indicator solution is added to each solution. [pH meter is optional] These solutions provide a reference for students to determine the pH of the solutions used in the demonstrations. The solutions in the demonstration have a few drops of Universal Indicator solution added.
A large sample of deionized or distilled water is adjusted to pH = 7.0. Universal indicator solution is added. A third of this solution is placed in Erlenmeyer flask "A". Erlenmeyer flask "A" and its contents are on a stir plate with a magnetic stir bar in the water. Small quantities of 010 M HCl and then 0.10 M NaOH are added to water. Students observe the color of the indicator in the water changes dramatically. Have students make note of this but do not say anything else at this point in the demonstration. Do not say, "Water is not a buffer solution".

Step 2: Place a third of the deionized water in Erlenmeyer flask "B". Erlenmeyer flask "B" and its contents are on a stir plate with a magnetic stir bar in the water. Tell students you are going to add acetic acid.
The instructor asks students to estimate the pH of the solution when acetic acid is added. "Will the pH become more acidic, less acidic, or remain the same?"
Ask students to write an equilibrium chemical equation representing this weak acid system.
CH3COOH(aq) + H2O(l) <=> H3O+(aq)+ CH3COO- (aq) Ka = 1.8 x 10-5
Add acetic acid to the water. Students observe a color change from "green" (neutral) to "orange/red" (acidic). Students observe the indicator in the acetic acid solution has a color corresponding to an acidic solution, a weak acid solution.
Ask students to draw a particle level diagram representing the equilibrium of this weak acid system.
[diagram inserted here]
Step 3: Place a third of the deionized water in Erlenmeyer flask "C", Erlenmeyer flask "C" and its contents are on a stir plate with a magnetic stir bar in the solution. Tell students you are going to add solid sodium acetate to the water. The instructor asks students to estimate the pH of the sodium acetate solution and to write an equilibrium chemical equation representing this salt system. Kw = Ka Kb
CH3COO- (aq) + H2O(l) <=> CH3COOH(aq) + OH-(aq) Kb = Kw/ Ka = 1.0 x 10-14/ 1.8 x 10-5 =
Add solid sodium acetate to the water. Students observe a dramatic color change from green to blue. The indicator in the acetate solution has a color corresponding to an alkaline solution, a weak base solution.
Ask students to draw a particle level diagram representing the equilibrium of this salt system.
[diagram inserted here]
Step 4: The instructor asks students to write a balanced chemical equation to represent what happens when "sodium acetate is added to the acetic acid?" Do not say anything else. Allow time for students to attempt to write a chemical equation. You can tell students that Na+ is a spectator ion. Most students will have difficulty writing a balanced chemical equation because students will write "acetic acid reacts with sodium acetate". Acetic acid and the acetate ion are represented as reactants in their chemical equation.
an incorrect chemical equation: CH3COOH(aq) + CH3COO- (aq) <=>
This presents a difficulty for students because to balance the equation students write
CH3COOH(aq) + CH3COO- (aq) <=> CH3COOH(aq) + CH3COO- (aq)
Tell students to leave their chemical equation as is for the moment.
Ask, "what will happen to the pH of the acetic acid solution when the solution of sodium acetate is added. Will the pH become more acidic, less acidic, or remain the same?"
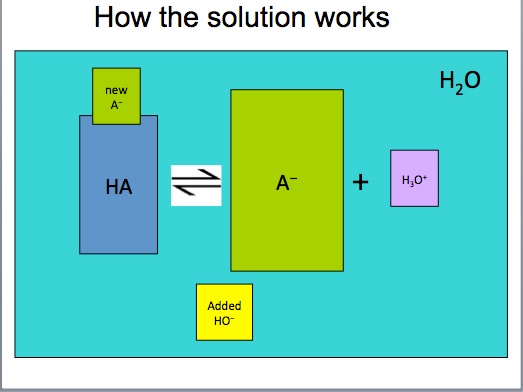
Students should invoke LeChatelier's Principle and the common ion effect. The acetate ion is the common ion. When added to the acetic acid system at equilibrium, the acetate will react with some of the hydronium ions, causing the equilibrium to a shift to the left. Since the hydronium ion concentration decreases, the pH should increase (become less acidic). Do not mention or say the word "buffer". Save this discussion until after the demonstration.
Next, solid sodium acetate solution is added to the acetic acid solution. The color of the indicator in the solution should be "yellow" corresponding to pH = 5. If some of the original water remains, point out to students that water started out as neutral. The acetic acid/acetate solution is not neutral. The solution is slightly acidic.
Ask students to draw a particle level diagram representing the equilibrium of this solution.
[diagram inserted here]
Small quantities of 010 M HCl and then 0.10 M NaOH are added to the acetic acid acetate solution. Students observe the color of the indicator in the solution changes a bit for a second then adjusts to the "yellow" color.

Have students summarize what they have observed.
- The pH of water changes dramatically when small amounts of acid or base are added.
- When small amounts of acid or base are added the acetic acid/acetate solution, the solution resists changing the pH.
An acidic buffer is a solution of a weak acid (acetic acid) and its conjugate base pair (sodium acetate) that prevents the pH of a solution from changing drastically through the action of each component with incoming acid or base.
Web page author: T. Greenbowe, University of Oregon. This page is under construction.
Acetic acid in the buffer solution will react with the addition of sodium hydroxide, NaOH
CH3CO2H(aq) + OH-(aq) –> CH3CO2-(aq) + H2O(l) K = 1.8 x 109
The acetate anion in the buffer solution will react with the addition of hydrochloric acid, HCl
CH3CO2-(aq) + H3O+(aq) –> CH3CO2H(aq) + H2O(l) K = 5.6 x 104
The following URL has a didactic lecture demonstration video about comparing water to an acetic acid/acetate buffer solution. The Video on the ACS web site credits McGraw-Hill.
Several 250 mL Erlenmyer flasks or large beakers depending upon the number of students in the class.
Universal Indicator solution, Deionized water
A white back ground board and white paper to put underneath the demonstration area (to improve visibility)
Solutions pH = 4, 5, 6, 7, 8, 9, 10 in labeled Erlenmeyer flasks with universal indicator in each solution.
Three stir plates, three magnetic stir bars, plastic droppers, two small beakers labeled 0.10 M HCl, 0.10 M NaOH
3M or 6 M acetic acid, 50 g of solid sodium acetate, 0.10 M HCl, 0.10 M NaOH,
Waste container
A pH meter is optional.
Discussion
Ask students to predict the pH of this system. Nearly all students will say, with confidence, acidic. Add some universal indicator solution and students will observe the color of the indicator corresponds to an acidic solution. Tell students the acetic acid is the "Big Dog" in the yard. The "Big Dog" establishes the equilibrium system.
Next ask students what will happen to the pH of the solution when some solid sodium acetate is added to the acetic acid solution? Tell students that the sodium acetate is the "Little Dog". Remind students they have observed the common ion effect and they know Le Chatelier's Principle. Students should predict that adding some acetate ions will shift the equilibrium to the left, decreasing the [H+] concentration, thus increasing the value of pH.
Suggested Video Lecture URLs
The following animations present a simplified representation of a molecular view of what occurs when acid or base is added to a buffer system.
http://www.chembio.uoguelph.ca/educmat/chm19104/chemtoons/chemtoons7.htm
https://youtu.be/ZLKEjXbCU30 (accessed July, 2023)
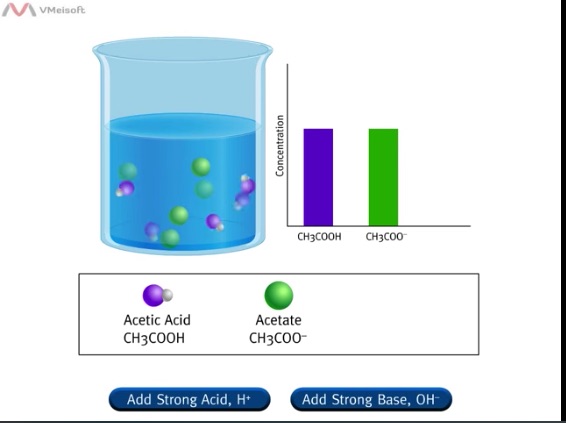
Suggested Computer Animation
Ask students to write the equilibrium equation representing this system. Some students will attempt to write
HA + A- <=>
Students will need to be convinced that the equation representing the equilibrium system remains
HA + H2O <=> H+ + A-
Acetic acid is the "Big Dog" and the "Big Dog" establishes the equilibrium in the yard. For a complete explanation of how to incorporate the "Big Dog" see xxx.
Extensions
Buffer Capacity
Is an indication of how much added acid or base will be neutralized by the components of the buffer solution. Buffer capacity depends on the concentration of weak acid and the concentration of the the conjugate base. The more concentrated the components of a buffer, the greater the capacity to resist changes to pH.
Buffer Range pH = pKa ± 1
Buffer range is the pH range over which the buffer solution will effectively buffer - neutralize incoming acid and or base. Buffer solutions are most effective when the pH range = pKa ± 1
Making a Buffer Solution with a Target pH Henderson-Hasselbalch Equation
Particle Level Representation
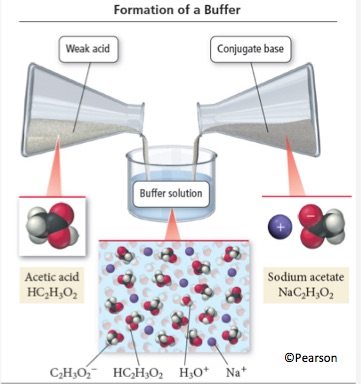
Figure from Tro, N. (2022). Chemistry: A Molecular Approach 6th edition. Pearson: Hoboken, NJ.
Curriculum Notes
A Class activity accompanies this interactive demonstration. A computer animation showing a dynamic representation of the interactions of weak and and conjugate base in the the acid-base reactions at the particle level can accompany this activity. When instructors expect their students to think of buffers in microscopic or symbolic terms and to relate the different representations of buffers with each other, they need to show those representations and their connections with the students explicitly. Students do not generally make these connections on their own or think in terms of "molecular scenes".
Clicker questions asking students to predict what will occur can accompany this activity. Quiz questions assess students understanding of buffer solutions are available. Sample lecture notes accompany this activity.
Student Difficulties
1. Buffer solutions are commonly viewed by students as static systems instead of dynamic equilibria systems.
2. Students have a difficult time interpreting chemical formulas confidently. Students have difficulty with identifying if chemical formula represents a weak or strong acid, weak or strong base, acidic or basic ionic salt. The chemical formulas for salts of conjugate bases are particularly hard for students to interpret.
3. Students have a difficult time understanding and predicting if a a soluble ionic salt will generate an acidic, basic, or neutral solution when dissolved in water.
4. Students have a difficult time writing an equilibrium chemical equation representing a buffer system. Students not understand the relationship between a weak acid and its conjugate base. Students assume that any two chemicals that are mixed will react together and students will write an equation for the chemical reaction between the weak acid and its conjugate base.
HA + A- <=>
5. Student difficulties in understanding buffer conceptually are related to their inability to visualize buffers on the microscopic scale. Students have a difficult representing (drawing) a buffer solution using a "picture diagram" or "molecular scene" and have difficulty interpreting a "molecular scene" representing of a buffer . Students have difficulty relating the macroscopic, microscopic and symbolic representations of buffers. Students who can draw and interpret "molecular scenes" of buffer solutions exhibit and demonstrate a better conceptual understanding of buffers compared to students who cannot do so.
6. Students confuse the concentration of hydronium ion in the buffer solution (used to calculate the pH) with the initial concentration of weak acid (i.e. 1.0 M) - used in the Henderson-Hasselbalch equation. Students are not able to distinguish between or relate the weak acid component of the buffer and the hydrogen ions that determine the pH of the solution.
7. Students believe that all buffers have a pH = 7, neutral.
8. Students do not conceptual relate that fact that pH is a logarithmic scale and the log of the concentration of the H3O+ ions in solution (with acknowledgement of the role that an activity coefficient plays) determines the acidity or alkalinity of the solution. Students’ inability to understand logarithmic functions (base 10) has consequences for their understanding of buffers.
9. Students have difficulty differentiating between Ka and pKa or pH and [H+].
10. Because students do not have a good conceptual understanding of buffers and because they try to approach buffer problems from a purely mathematical perspective, some students believe that there is only one way to solve a particular type of buffer problem.
11. Some students believe the strength of the buffer is determined by the strength of its component acid and base: a buffer made from a strong acid and a strong base would be stronger (have a higher buffer capacity) than a buffer made from a weak acid and a weak base.
12. Some students have the idea that buffers have an unlimited ability to resist pH changes. In fact, buffer solutions have a finite capacity to resist pH changes. In an acidic buffer solution it is the number of moles of weak acid and number of moles of its conjugate base that determine the extent to which a buffer can neutralize added acid or base.
13. Students have a difficulty explaining what occurs when strong acid or a strong base is added to a buffer system. Students need to incorporate in their explanation, chemical equations, particle diagrams, and written explanations.
1. Identify two components of an acidic buffer solution and explain the function of each component.
2. Write the chemical equilibrium equation representing and acetic acid-sodium acetate buffer system.
3. Write appropriate chemical equations and explain how one component of a buffer system reacts when acid is added, and the other component reacts when base is added. Show that these reactions only slightly increase or decrease the pH of the solution.
4. Write appropriate chemical equations and explain how water reacts when acid is added, and when base is added. Show that these reactions increase or decrease the pH of the solution.
5. Write appropriate chemical equations and explain why the concentrations of the two buffer components must be high to minimize the change in pH due to the addition of small quantities of acid,H3O+, or base OH-.
6. Explain why the best pH of an acidic buffer system is ±1 pH of the pKa of the weak acid.
AP Chem Learning Objective
The student can identify a solution as being a buffer solution and explain the buffer mechanism in terms of the reactions that would occur on addition of acid or base.
References
Orgill, M.K., & Sutherland, A. (2008). Undergraduate chemistry students’ perceptions of and misconceptions about buffers and buffer problems. Chemistry Education Research and Practice, 9, 131–143.
Drechsler, M., & Schmidt, H. J. (2005), Textbooks’ and Teachers’ Understanding of Acid-Base Models Used in Chemistry Teaching, Chemistry Education Research and Practice, 6 (1), 19-35.
Summerlin, L.; Borgford, C.; Ealy, J. Chemical Demonstrations: A Sourcebook for Teachers; Volume 2; 1987; p. 172-173.
Tro, N. (2022). Chemistry: A Molecular Approach. 6th edition. Pearson: Hoboken, NJ.
Donahue, C.J.; Panel, M.G. (1985). Buffer capacity of various acetic acid-sodium acetate systems: A lecture experiment. Journal of Chemical Education, 62(4) p 337. DOI: 10.1021/ed062p337.
Paul G. Hobe Jr. (1979). Buffer effect demonstration on the overhead projector. J. Chem. Educ., 56 (1), p 47.
James C. Chang (1976). A buffer solution and its action. J. Chem. Educ., 1976, 53 (4), p 228.
