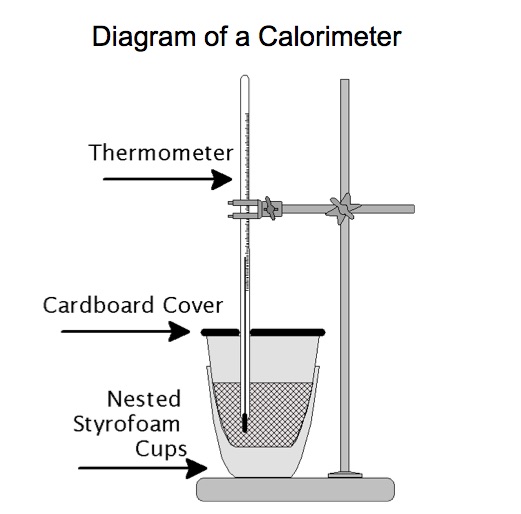
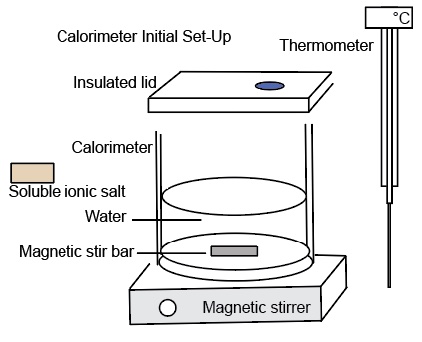
A calorimeter is a device consisting of an insulated container, a lid, a hole in the lid for a thermometer. A paddle wheel or magnetic stirrer is used to ensure mixing of substance. Placed in the calorimeter initially is a liquid, usually water. Figure 1a and 1 b shows a digram of the components of a calorimeter.
 |
|
Insulation and a lid prevents thermal energy from being transferred outside the calorimeter. Measuring the mass of the objects to be placed in the calorimeter, the initial and final temperatures, and the specific heats of the objects allows one to determine the energy exchanged during a physical or chemical process.
Calorimetry depends on three laws of thermodynamics. The first law of thermodynamics incorporates the law of conservation of energy. When placing a hot metal in cool water one can use an application of the Law of Conservation of Energy to model what occurs with respect to energy. The total energy change of a system ∆E system = Work + Q In the case of a calorimeter, a closed system, no work is done.
We can associate, q, as a calculated amount of thermal energy transferred. Thermal energy is defined as the average kinetic energy of the atoms and or molecules in an object.
q released by the hot object + q gained by the cool object = 0
Web page Author: Tom Greenbowe, University of Oregon. This page is under construction
In this calorimter experiment, there is an exchange of thermal energy. The hot object will transfer thermal energy to the cool object. Under ideal conditions, there is no net loss or gain in energy. The thermal energy released by the hot object is the thermal energy gained by the cool object. This exchane is due to collisions of atoms (in the case of two metal blocks placed in contact wiht each other). Befor contact, the atoms in the hot metal are vibrating faster compared to the atoms in the cool metal.
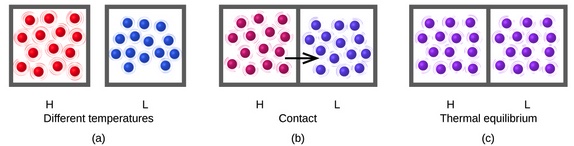
During contact the surface metal atoms in the hot metal collide with the surface metal atoms of the cool metal atoms. The vibrations of the hot metal atoms decrease and the vibrations of the cool metal atoms increase. When the two metal blocks are at the same temperature, the average vibrations of the metal atoms in the two blocks are similar.
Remember, heat is not a type or form of energy.
Thermal energy released is defined as a negative quantity because when an object or system releases heat energy, in the end it has less energy. Thermal energy gained by an object or system is defined as a positive quantity because when an object or system gaines thermal energy, in the end it has more energy compared to when the process started.
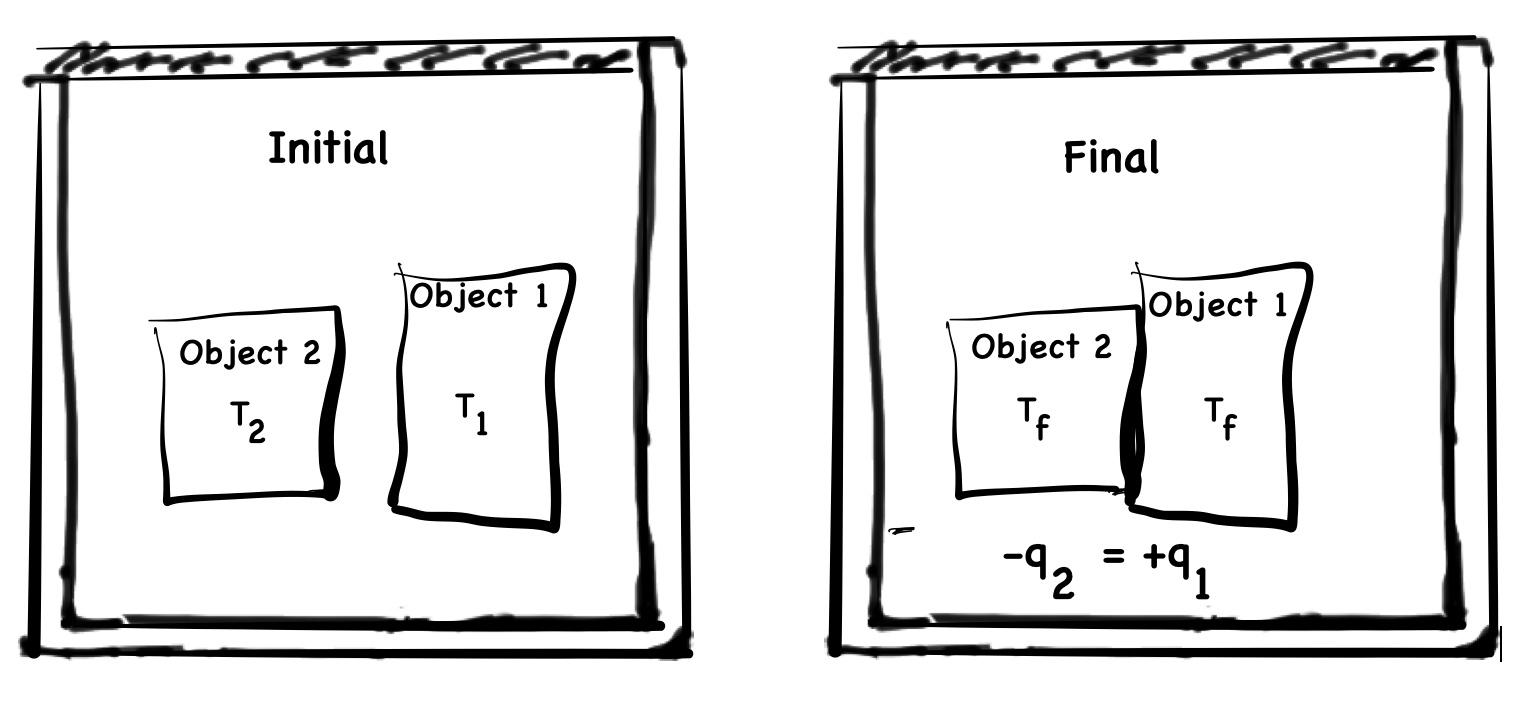
Figure 2 shows two objects at different temperatures initially separated in a calorimeter. The two objects are brought together. Thermal energy, q, is transferred from the hotter object, q2, to the cooler object, q1, until the same temperature is reached. When the two objects are at the same temperature, there will not be a change temperature. This is known as thermal equilibrium.
Placing a hot metal in contact with a cool metal: A particle view
https://www.youtube.com/watch?v=_5AZwrTkQNA
[explanation here]
Not all particles (atoms) have the same vibrations at a specific temperature. There is a range of vibrations.
Dissolving an ionic salt in water
Dissolving an ionic salt in water is a complex process. For the purpose of this presentation, we will assume that the dissolving process has aspects of both a chemical process (intermolecular forces are disrupted, energy is absorbed in order to pull apart ions in a lattice, ion-dipole interactions form, new chemical species form, there is a release or absorption of energy, and in most cases there is a change in the pH of the solution) and a physical process (evaporating the solution leads to recovery of the original salt). The dissolving process involves bond breaking, disruption of hydrogen bonding, and formation of ion-dipole interactions. Chemical bonds (ion-ion), intermolecular forces, and interactions have no mass and have no association with temperature. Breaking and forming chemical bonds involves changing the potential energy of the species involved in the dissolving process (water molecules and ions). A chemical equation has no mass. A chemical reaction has no mass and no temperature. One cannot not directly see a chemical reaction - with respect seeing bonds breaking and bonds forming. The "dissolving process" has no mass and no temperature. One cannot directly see a dissolving process - with respect seeing bonds breaking and bonds forming. We measure the mass, initial and final temperature of the solution. The bulk chemicals - the solid salt and the water - are not the dissolving process. Seeing solid salt disappear into water is an observation and we often say we "see the solid dissolving in the water". However, individuals cannot see the actual "dissolving process". If a dissolving process releases energy, the resultant solution gains this energy. We calculate the thermal energy absorbed by the solution, q solution. The thermal energy absorbed by the solution, when LiCl dissolves in water, is a positive quantity.
Using a coffee-cup or thermosbottle style calorimeter for conducting an experiment on a dissolving process, one observes an increase in temperature of the resultant solution. We can calculate the thermal energy gained by the resultant solution. A complicating factor is the dissolving process occurs within the solution. We need to keep the two processes separate in our thinking and our calculations.
q solution = mass x specific heat x change in temperature
The Law of Conservation of Energy states energy cannot be created or destroyed. Remember, heat is not a type (or form) of energy of energy. In every calorimetry experiment something releases thermal energy and something gains this thermal energy. This is the big idea for calorimetry and must be emphasized to students multiple times. If a dissolving process releases thermal energy then the solution absorbs this thermal energy. If we calculate the thermal energy absorbed by a solution, q solution, we infer the thermal energy released by a dissolving process, q dissolving process, is equal but opposite in sign. This is an application of the Law of Conservation of Energy.
q solution + q dissolving process = 0
Identify What Releases Thermal Energy and What Gains Thermal Energy
In a calorimeter experiment, it is better to have students first identify what releases thermal energy and what gains thermal energy. After students do an analysis of the experiment, then identify a system and surrounding. When lithium chloride dissolves in water in a calorimeter the dissolving process releases thermal energy and the resultant solution gains thermal energy.
Energy is transferred during a dissolving process from several different sources. There is a net change in enthalpy, ∆H, of the dissolving process.
∆Hdissolution = ∆Hsolution = H reactants - H products
For a process that releases energy, the enthalpy of the products are lower compared to the enthalpy of the reactants. The reactants have stronger bonds compared to the products. In the case of dissolving solid lithium chloride, the ion-ion interactions of the LiCl in the solid and the hydrogen bonding IMFs of water are stronger compared to the ion-dipole interactions, hydrogen bonding interactions, and ion-ion interactions of ions and molecules in the resultant solution. Bond breaking, bond forming, and ion-dipole interactions forming and breaking are changes in potential energy.
The potential energy released by the dissolution process is transformed (or converted) to an increase in kinetic energy of the molecules and ions in the solution.
Dissolving lithium chloride in water results in a net release of potential energy by the chemical bonds and ion-dipole interactions. Overall, dissolving lithium chloride in water is an exothermic process. By the dissolving process we mean bonds breaking and forming and interactions breaking and forming. The heat of solution, change in enthalpy of solution, ∆Hsolution, is negative. Technically, this is often called the change in enthalpy of dissolution. Unfortunately, ∆Hsolution is also called the heat of solution or heat of dissolution.
Physics
With respect to the physics associated with the dissolving process, there are three main factors to consider - changes in potential energy, changes in kinetic energy, and changes in entropy For an exothermic dissolving process there is a net lowering of the potential energy of the "system - defined as bonds and interactions" and there is an overall increase in the kinetic energy of particles (molecules and ions) in the resultant solution. Overall, potential energy is converted (or transformed into) to kinetic energy of vibrating atoms when bonds break. In the case of lithium chloride solid, the ions are vibrating moderately in the solid. When ion-ion forces break, the decrease potential energy is transformed to an increase in kinetic energy of the ions - the ions vibrate vigorously. There is also an increase in entropy in the LiCl dissolving process. There are more microstates created after the solid dissolves in water to form a solution.
xx
vv
animation
[add]
References