A Calorimetry Computer Simulation is used to determine the heat energy exchanged in a variety of physical and chemical processes. This computer simulation allows one to select the mass and initial temperature of various substances, mix the substances in a calorimeter, and record the final temperature. About 125 different experiments can be performed using this simulation:
Experiments
placing hot metal in cold water mixing hot and cold water placing hot metal in ethanol mixing ethanol and water
heat of neutralization - mixing various acids and bases (strong acid + strong base, weak acid + strong base, etc.)
heat of dissolution - dissolving ionic salts in water
This simulation and embedded animations can be used to accompany a variety of calorimetry demonstrations and in-class activities. The simulation can be used for a variety of laboratory experiments or to accompany calorimetry experiments. Students can ask a research question, design an experiment to answer the question, run the experiment, collect data, and analyze results. The computer simulation is designed to be used with a tutorial or guided-inquiry activity - the simulation is not designed as a stand-alone activity.
Web-page author: T. Greenbowe, University of Oregon. This page is under construction.
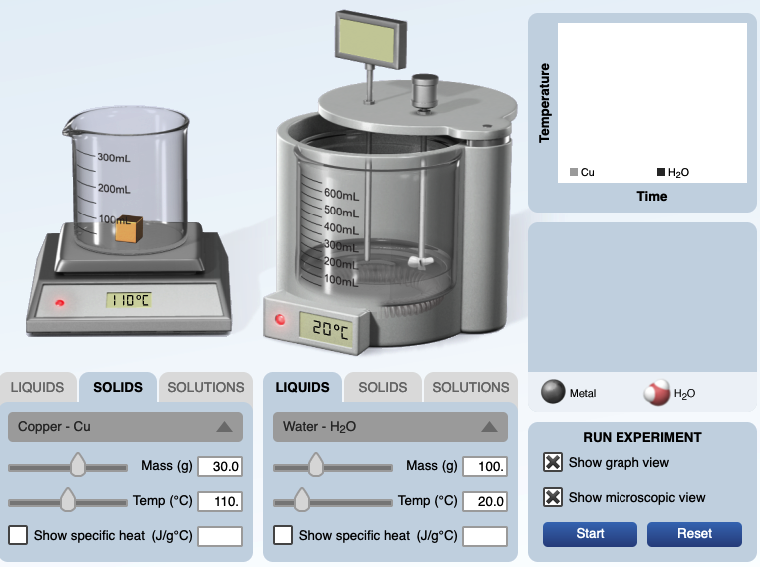
Example. In the calorimeter experiment below, 30.0 grams of copper metal at 110.°C is ready to be placed in 100. grams of water at 20.0°C.
Greenbowe, T.J., Gelder, John I., Abraham, M.R. (2017). Calorimetry Computer Simulation. Pearson Education, Inc.: Hoboken, NJ.
Currently Firefox 112.1 is able to play the animations (April, 2023).
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
 |
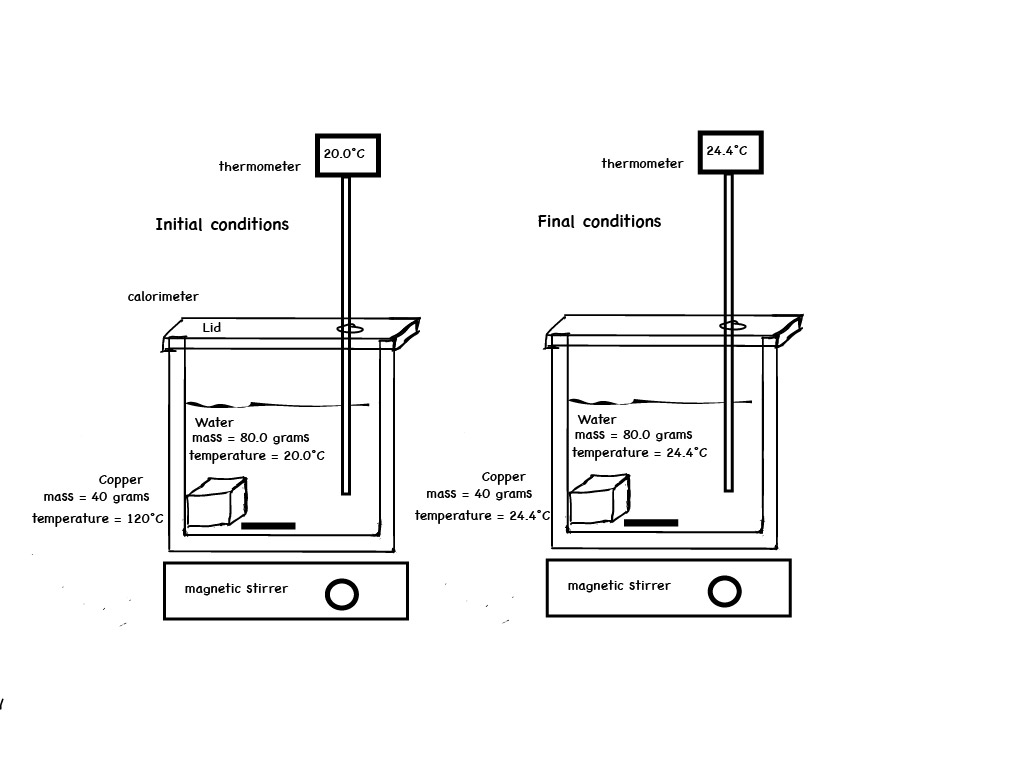
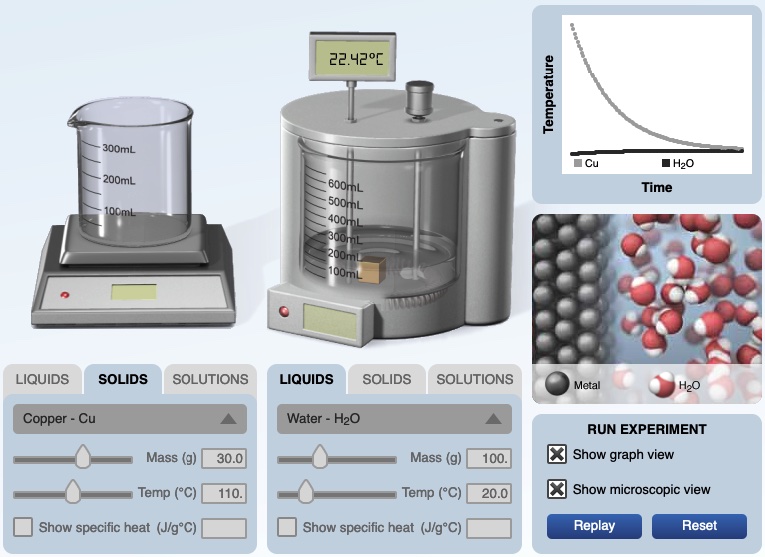
The final temperature of the copper metal and water is 24.42°C. A graph of temperature versus time indicates the temperature of the water decreased and the temperature of the copper metal increased. The specific heat of copper can be determined.
The big idea in all calorimetry experiments is that something will release heart energy and another entity will gain this heat energy. The First Law of Thermodynamics states, in part, that energy is conserved in all processes - the Law of Conservation of Energy. For a local process we use "q" to represent heat energy exchanged
We can use the formula q loss + q gain = 0
In a calorimeter, the hot metal released energy and the cool water gained this energy.
An animation of the energy exchange
The animation in the computer simulation is a simple model and it has limitations. The animation focuses on the kinetic energy of the particles and does not include the contribution of potential energy. When the hot copper metal is added to the cool water, an animation representing the copper atoms and water molecules provides a visualization of how energy is transferred. Initially, the hot metal atoms vibrate vigorously and the water molecules move slowly. The cool water molecules collide with the hot copper atoms at the surface of the metal. This collision transfers kinetic energy. The hot copper atoms decrease in energy (less vigorous vibrations) and the cool water molecules increases in kinetic energy. The more vibrant colored water molecules move faster compared to the original movement of water molecules prior to the placement of the hot copper metal in the water. The temperature of the water increases due to an increase in velocity of the water molecules. The water molecules strike the thermometer with more force, the thermometer indicates a higher temperature. The temperature of the metal decreases due to less vigorous vibrations of the metal atoms. This process continues until thermal equilibrium is achieved.
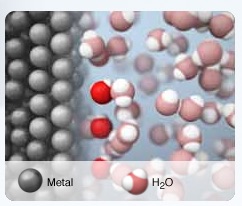
Other metals can be selected to investigate. Ask students, "for equal masses of hot metal placed in the same mass of water, which metal raises the temperature of water the most?"
This versatile computer simulation can be used as part of a lecture presentation, POGIL classroom activity, as a component of a laboratory experiment involving calorimetry, and thermochemistry, as an enhancement of lecture demonstrations, as a make-up laboratory experiment, as part of an end-of-chapter homework assignment, etc.
The simulation has a particle view (molecular scene) animation of the ions, atoms and or molecules interacting during the reaction or process. This provides the opportunity for students to view a representation of how energy is exchanged during a chemical reaction. Animations for the reaction of acids and bases, as well as dissolving ionic salts in water are available.
Computer animations of the physical process or chemical reaction at the particle level of representation (molecular scenes) are provided for a select few of the experiments. By combining this simulation with a demonstration or experiment and a guided-inquiry activity, students will be able to work with the three levels of representation: macroscopic, microscopic, and symbolic as recommended by A. Johnstone (1982, 1993).
Acid-Base Neutralization Reaction
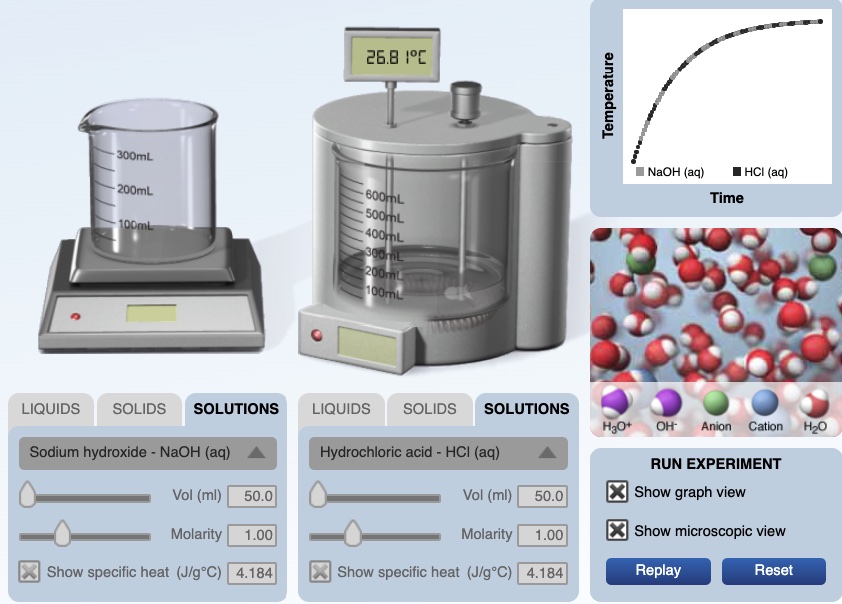
For example, when HCl(aq) and NaOH(aq) react, the chemical reaction releases energy and the resultant solution, NaCl(aq), gains energy. In a calorimetry experiment involving reacting acids and bases, we measure the initial temperature of the two substances, HCl(aq) and NaOH(aq) and the mass of the two substances. We can measure the mass of the resultant solution and the final temperature of the resultant solution, NaCl(aq) and H2O(l). In a strong acid strong base reaction, the temperature of the resultant solution increases. We need to know the specific heat of the NaCl(aq) solution. Often it is assumed the specific heat is 4.18 J/g°C, but an actual value can be used.
The heat gained by the resultant solution is calculated using
q solution = mass solution x specific heat solution x (T final solution - T initial solution)
In this experiment, we are using a well insulated calorimeter with a lid containing a closed system. We assume no heat energy leaves or enters the calorimeter. The Law of Conservation of Energy applies to this experiment. In every calorimeter experiment, something releases heat energy and something gains heat energy. In this experiment, the chemical reaction, defined as bonds breaking and bonds forming, releases heat energy, q reaction . The resultant solution gaines heat energy, q solution .
The heat released by the reaction is calculated using q reaction + q solution = 0 q reaction = - q solution
When HCl(aq) reacts with NaOH(aq) an animation representing the ions and water molecules provides a visualization of how energy is transferred. The animation focuses on the kinetic energy of the particles and does not include the contribution of potential energy. Initially, the ions and the water molecules move slowly. A hydronium ion collides with a hydroxide ion to form two new water molecules. When this occurs potential energy converts to kinetic energy. The newly formed water molecules move fast.
H3O+(aq) + OH-(aq) --> 2 H2O(l) ∆H = -52 kJ/mole
This collision transfers kinetic energy. The new water molecules increase in movement (more kinetic energy). These more vibrant colored water molecules strike other slower moving water molecules, which increases their movement (more kinetic energy). The temperature of the water increases due to an increase in velocity of the water molecules and ions. The water molecules and ions strike the thermometer with more force, the thermometer indicates a higher temperature. The temperature of the resultant solution increases.
A the end of the experiment, all of the HCl(aq) reacts with the NaOH(aq) to form aqueous sodium chloride and water. The final temperature of solution is 26.8°C. A temperature versus time graph shows that the solution increased in temperature.
Calorimetry Computer Simulation HTML5 Version
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
©2017 Greenbowe, T., Abraham, M. Gelder, J. Calorimetry Computer Simulation, Pearson: Hoboken, NJ.
Hess' Law experiments
Curriculum Notes
In all calorimetry experiments something releases energy and something gains energy. In the case of the acid-base reaction, the chemical reaction released energy and the resultant solution gained energy. The big idea for all calorimetry processes is energy is conserved. Energy cannot be created or destroyed, but it can be exchanged.
qlost + qgain = 0 or qreleased + qgain = 0
Students experience difficulty learning thermochemistry concepts. Determine the heat energy exchanged by the solution, at constant pressure. We know the mass of the solution, the specific heat of the solution, and the change in temperature of the solution.
q solution = m solution x c solution x ∆Tsolution
The heat energy exchanged by the reaction can be inferred. q solution + q reaction = 0
Calculating the limiting reactant, the change in enthalpy of the reaction, ∆Hrxn, can be determined since the reaction was conducted under conditions of constant pressure
∆Hrxn = qrxn / # moles of limiting reactant ∆Hrxn is then reported with respect to the reaction as written.
This computer simulation can be used to illustrate how the amount of reactants reacting influences the heat exchanged, q, but does not influence ∆H.
Heat energy is transferred between a system and a surrounding as an interaction. Heat energy is not a property of a system. Heat energy is not an object nor is it a substance. In this experiment, kinetic energy is transferred when faster vibrating (or moving) atoms or molecules collide with slower vibrating (or moving) atoms or molecules. On average the collisions cause the faster vibrating atoms to loose kinetic energy and the slower vibrating atoms to gain kinetic energy. This process of energy exchange at the atom level is called thermal interaction or thermal energy. In the case of the acid-base reaction, there is an increase in kinetic energy. Where does this energy come from? During an acid-base chemical reaction chemical bonds are broken and chemical bonds are formed. It takes an input of energy to break a bond. When bonds form, there is a release of energy. This energy is potential energy. For an exothermic reaction, there is a net lowering of the potential energy of the system. The change in potential energy is converted to kinetic energy.
Temperature. Quantifies the "hotness" or "coldness" of an object relative to a standard. Temperature is a state variable. A thermometer measures temperature in a localized area of an object.
Thermal energy. Energy of an object due to motion of atoms, ions, and molecules. Thermal energy is a state variable. Thermal energy is not heat.
Thermal equilibrium. Two objects are in thermal equilibrium when in contact and the temperature is the same for both objects.
Learning Objectives
1. Use experimental data to develop a conceptual understanding of the First Law of Thermodynamics and how to apply it to calorimeter experiments:
q lost + q gain = 0
2. Ask a research question and design a series of experiments to provide data to answer the research question.
3. Use experimental data to develop a relationship among the variables: heat, mass, specific heat, and change in temperature.
4. Identify whether the process is exothermic or endothermic.
5. Identify what gains heat and what loses heat in a calorimetry experiment.
6. For a physical process and for a chemical reaction explain how heat is transferred, released or absorbed, at the molecular level.
7. Calculate the heat gained or released by a solution, qsolution, involved in a given calorimetry experiment: total mass of the solution, specific heat of the solution, change in temperature of the solution: q = m c ∆T
8. If the calorimetry experiment is carried out under constant pressure conditions, calculate ∆H for the reaction.
9. Given the change in enthalpy for a reaction, the amounts of reactants, and a balanced chemical equation, calculate the heat exchanged for a reaction.
10. Show that the amounts of reactants that react influences q, the heat exchanged during an acid-base neutralization reaction, but does not influence, ∆H for the reaction.
AP Chem Learning Objectives
Learning objective 5.4 The student is able to use conservation of energy to relate the magnitudes of the energy changes occurring in two or more interacting systems, including identification of the systems, the type (heat versus work), or the direction of energy flow.
Learning objective 5.5 The student is able to use conservation of energy to relate the magnitudes of the energy changes when two nonreacting substances are mixed or brought into contact with one another.
Learning objective 5.6 The student is able to use calculations or estimations to relate energy changes associated with heating/cooling a substance to the heat capacity,
Making this computer simulation interactive - active learning
The instructor should "frame" the instructional activity accompanying this computer simulation. Use one of the calorimetry POGIL activities.
Students have difficulties with several concepts associated with thermochemistry (see reference #1 below).
Students do not have difficulty with the idea that the amount of reactants influences the heat exchanged, but they do have difficulty applying this concept.
There are several calorimetry lecture demonstrations that can be used to accompany this computer simulation.
There are several in-class POGIL-like activity to accompany this computer simulation.
There are a set of interactive guided-inquiry Power Point slides to accompany this computer simulation.
There are clicker questions that can accompany this computer simulation if this computer simulation is used in class.
Footnotes
References
Herrington D. G. (2011). The heat is on: an inquiry-based investigation for specific heat. Journal of Chemical Education, 88(11), 1558-1561.
Greenbowe, T, Abraham, M., Gelder, J. (2017). Calorimetry Computer Simulation. Pearson Education, Inc.: Upper Saddle River, NJ.
Greenbowe, T.J. and Meltzer, D.E. (2003). “Student learning of thermochemical concepts in the context of solution calorimetry.” International Journal of Science Education, 25(7), 779-800.
Russell, JW, Kozma, RB, Jones, J Wykoff, N Marx, J Davis (1997). Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts Journal of Chemical Education, 74 (3), 330.
Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Learning, 7: 75–83.
Johnstone, A. H. (1982). Macro- and micro-chemistry. School Science Review, 64, 377–379
