Two different metals, aluminum and lead, of equal mass are heated to the same temperature in a boiling water bath. The specific heat capacities of each metal is displayed to students: Al 0.903 J/g°C Pb 0.160 J/g°C
Which metal will raise the temperature of the 50 grams of water the most?
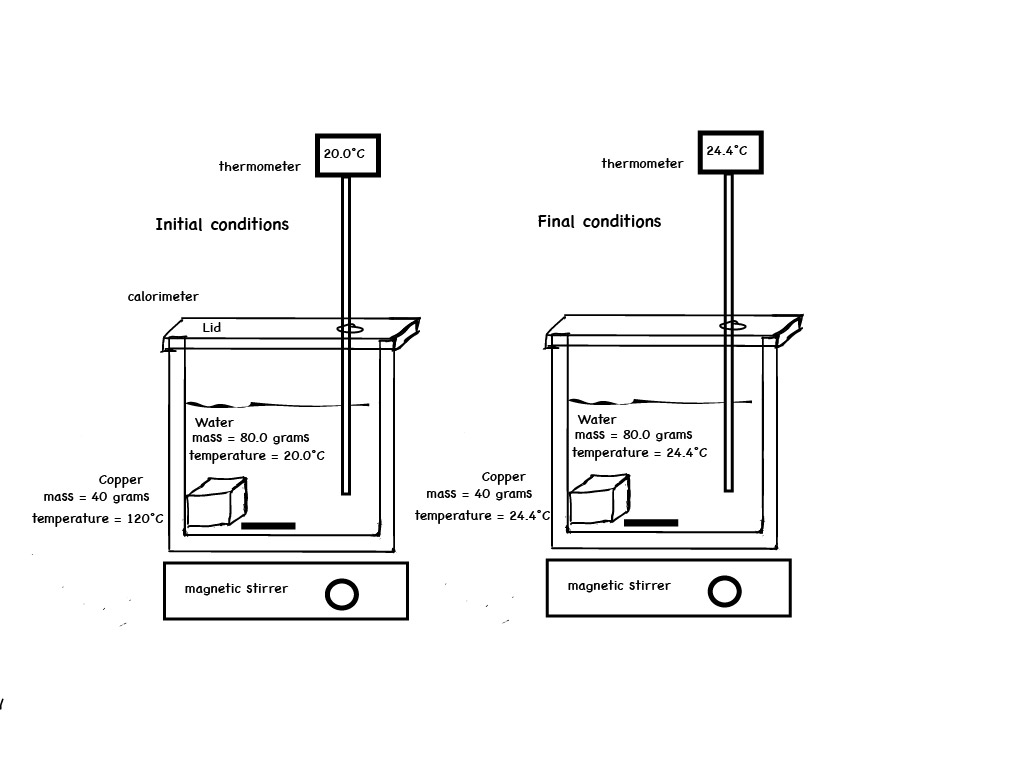
The metals are added to two insulated cups or calorimeters, each containing the same amount of water initially at room temperature. Students are asked to predict what will happen to the temperature of water and the temperature of the metals. The temperature of the water changes by different amounts for each of the two metals. For each experiment compare the heat gained by the cool water to the heat released by the hot metal. Compare the heat gained by the water in Experiment 1 to the heat gained by the water in experiment 2.
This demonstration assesses students' conceptual understanding of specific heat capacities of metals. A common misconception exhibited by some students follows this line of reasoning - "the metal that has the greatest temperature change, releases the most heat". In fact, given equal masses of metals starting at the same high initial temperature being placed in the same mass of cool water at the same initial temperature, the metal having the smallest temperature change releases the greater amount of heat to the water. Also, the metal with the higher specific heat will release more heat as it cools and thus will raise the temperature of water more compared to a metal with a lower specific heat.
If the accompanying computer animation is displayed students can gain a conceptual understanding of heat transfer between a hot sample of metal and the cool water at the particle level (atom level).
What do studenets observe?
Curriculum Notes
The Law of Conservation of Energy is the "big idea" governing this experiment. A natural transfer of heat or heat flow from a region of higher temperature to a region of lower temperature until a thermal equilibrium temperature is reached. In this demonstration, heat energy is transferred from a hot metal sample to a cool sample of water: qlost + qgain = 0
Each different type of metal causes the temperature of the water to increase to a different final temperature. This indicates that each metal has a different ability to absorb heat energy and to transfer heat energy.
The ability of a substance to contain or absorb heat energy is called its heat capacity. Heat capacity is an extensive property—it depends on the amount or mass of the sample. Specific heat is a measure of the heat capacity of a substance. Specific heat is defined as the amount of heat required to increase the temperature of one gram of a substance by one degree Celsius.
Specific heat: Al 0.903 J/g°C Pb 0.160 J/g°C
A calorimetry computer simulation can accompany this demonstration.
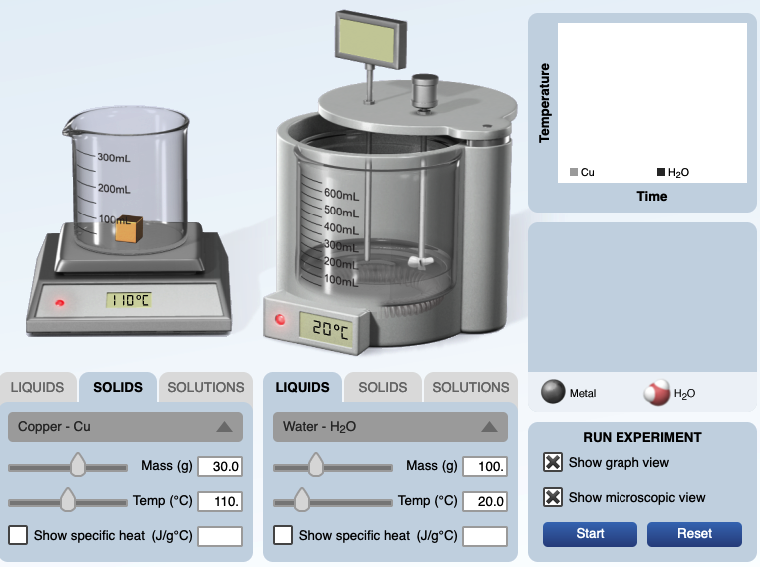
URL: https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
A computer animation depicting the interaction of hot metal atoms at the interface with cool water molecules can accompany this demonstration (see file posted on the side menu).
An in-class activity can accompany this demonstration (see file posted on the side menu).
A PowerPoint slide sequence of this presentation is included (see file posted on the side menu).
With some planning all three levels of representations of matter can be explored (not simultaneously) using ALEX JOHNSTONE'S triangle approach: macroscopic, microscopic, symbolic.
Web page author: T. Greenbowe, University of Oregon. This page is under construction.
Learning Objectives
1. Use experimental data to develop a conceptual understanding of temperature, heat, and specific heat capacities of metals. Be able to answer correctly conceptual questions involving temperature, heat, and specific heat capacities of metals.
2. Given appropriate calorimetry data for two metals, predict which metal will increase the temperature of water the most or the least.
3. Use experimental data to develop a relationship among the variables: heat, mass, specific heat, and change in temperature: q = m x c x ΔT
4. Given three of the four variables in q = m x c x ΔT determine the value of the fourth variable.
5. Apply the First Law of Thermodynamics to calorimetry experiments: qloss + qgain = 0
6. Identify what gains heat and what loses heat in a calorimetry experiment.
7. Explain how when the transfer of heat between a hot metal and cold water occurs in a calorimeter, the heat released by the hot metal is equal to the heat gained by the cool water, but the change in temperature of the metal is not the same as the change in temperature of the water.
8. For a physical process such as the transfer of heat between a hot metal and cold water, explain how heat is transferred, released or absorbed, at the molecular level.
9. Given appropriate calorimetry data for two metals, predict which metal will increase its temperature the quickest (shortest time) when each metal starts at room temperature and is uniformly heated.
Materials
Metals: Al and Pb, (and Zn) 100 g each
Digital thermometers, LapTop/PC with digital thermometer display
Hot plate
Balance, centigram (0.01-g precision) Insulated coffee cups, 6
Beaker, 600-mL Beakers, 400-mL,
Stirring rods, 3 Ring stand and clamp
Boiling stones Beaker tongs
1.0 L of Deionized Water ; Graduated cylinder, 100-mL
Procedure for Presenting an Interactive Demonstration
Because the density of aluminum is much lower than that of lead and zinc, an equal mass of Al occupies a much larger volume than Pb or Zn. Choose a large enough beaker such that both the aluminum metal and lead metal will be submerged in the boiling water bath. Heat the metals for about 6 minutes in boiling water. Place 50 mL of water in a calorimeter. Measure and record the initial temperature of the water in the calorimeter. Have students predict what will happen to the temperature of the water in the two calorimeters when hot lead is added to one and hot aluminum is added to the other. See the prediction clicker question posted on the right side menu (also see below for student results).
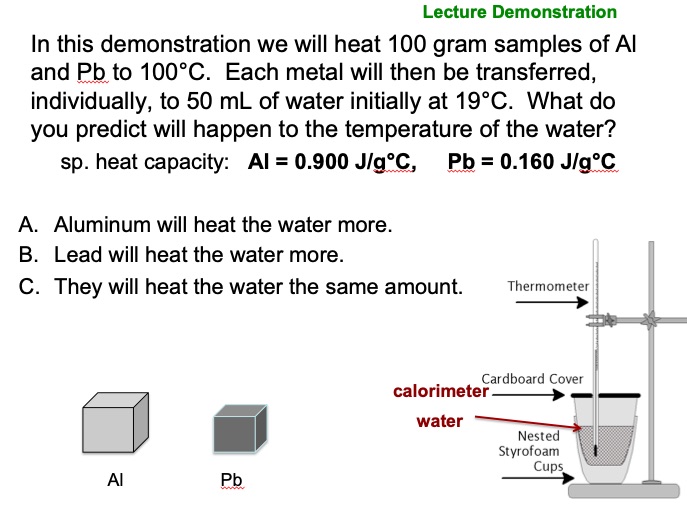
In this demonstration we will heat 100. g samples of Al and Pb to 100.°C. Each metal will then be transferred, individually, to 50. mL of water initially at ___°C. What do you predict will happen to the temperature of the water?
After students have answered the question, use the tongs and grab the hot lead metal and place it in 50 mL of room temperature water. Stir it up (Bob Marley). Record the final temperature of the water. Use the tongs and grab the hot aluminum metal and place it in the second calorimeter containing 50 mL of room temperature water. Stir it up. Record the final temperature of the water. Compare the final temperature of the water in the two calorimeters. Compare the change in temperature of the metals and the water. Compare the heat gained by the cool water to the heat released by the hot metal. Compare the heat gained by the water in Experiment 1 to the heat gained by the water in experiment 2 and relate this to the specific heats of the metals. Given a table of specific heats of four metals, have students answer the assessment question (posted on the right side menu and see below for student results):
Which 20.0 g sample of metal is most likely to undergo the smallest change in temperature upon absorption of 100.0 J of heat?
Sample Data and Sample Calculations
Mass | Initial Temp . | Final Temp. | Temp. Change | |
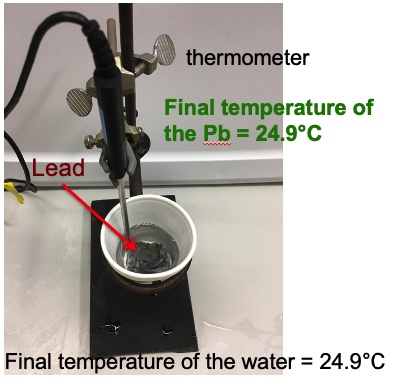
water | 50.0 g | 19.2°C | 24.9°C | +5.7°C |
lead | 100.0 g | 100.0°C | 24.9°C | -75.1°C |
q = m x c x ∆t
q lost Pb = 100. g x 0.160 J/g °C x (-70.0°C) = -1201 J
q gained water= 50.0 g x 4.18 J/g °C x (5.7°C) = +1191 J
Mass | Initial Temp . | Final Temp. | Temp. Change | |
water | 50.0 g | 19.2°C | 43.5°C | +24.3°C |
aluminum | 100.0 g | 100.0°C | 43.5°C | -56.5°C |
q gained water = 50.0 g x 4.18 J/g °C x (24.3°C) = +5078 J
q lost Al = 100.0 g x 0.900 J/g °C x (-56.5°C) = -5085 J
Within experimental error q gained by the water + q lost by the metal = 0
The heat released by the hot metal is equal to the heat gained by the cool water, but the change in temperature of the metal is not the same as the change in temperature of the water.
References and Literature Citations
Specific Heat A Chemistry Demonstration. In Thermochemistry—ChemTopic™ Labs Digital Collection. Flinn Scientific, Batavia, Illinois. 2016. https://www.flinnsci.com
Harrington, D.G. 2011. The Heat is on: An inquiry-based investigation for specific heat. Journal of Chemical Education, 88, 1558-1561.
Johnstone, A. H. 1993. The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9), 701-705.
Ita Cohen, Ruth Ben‐Zvi. Improving student achievement in the topic of chemical energy by implementing new learning materials and strategies. International Journal of Science Education, 1992, 14 (2) , 147-156. DOI: 10.1080/0950069920140203.
McCullough, T. 1980. A specific heat analogy. Journal of Chemical Education, 57(12), p. 896.
Johnstone, A. H. 1993. The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9), 701-705.
Ita Cohen, Ruth Ben‐Zvi. Improving student achievement in the topic of chemical energy by implementing new learning materials and strategies. International Journal of Science Education, 1992, 14 (2) , 147-156. DOI: 10.1080/0950069920140203.
McCullough, T. 1980. A specific heat analogy. Journal of Chemical Education, 57(12), p. 896.
Results of the Prediction Question Prior to Doing the Demonstration
In this demonstration we will heat 100. g samples of Al and Pb to 100.°C. Each metal will then be transferred, individually, to 50. mL of water initially at ___°C. What do you predict will happen to the temperature of the water?
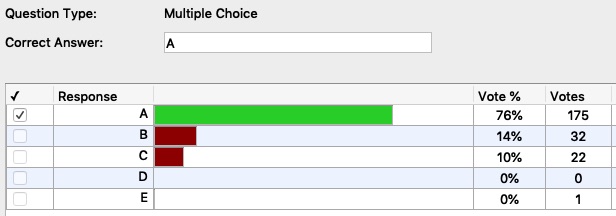
Results of the Assessment Question Immediately After Presenting the Demonstration
Given a table of specific heats of four metals, have students answer the assessment question: "Which 20.0 g sample of metal is most likely to undergo the smallest change in temperature upon absorption of 100.0 Joules of heat?"

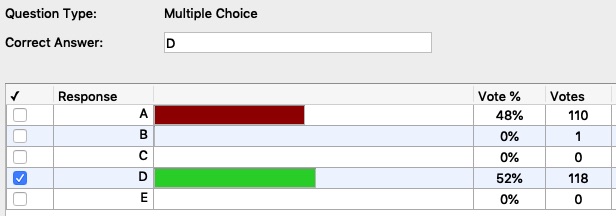
Mid-term Examination Questions and Results (Student Performance)
18. A 50.0 gram piece of heated copper is dropped into 50.0 grams of room temperature water. Which of the following statements is true? The specific heat of copper is 0.385 J/g°C and the specific heat of water is 4.184 J/g°C.
- The heat lost by the copper is equal in magnitude to the heat absorbed by the water.
- The final temperature of the copper will be equal to the final temperature of the water
- ΔT for the copper = - ΔT for the water
A. i only
B. i and ii only
C. iii only
D. i and ii only
E. i, ii and iii (all three are true)
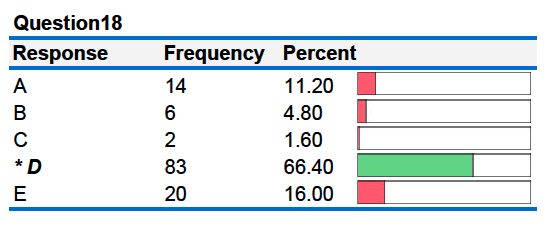
18B. A 50.0 gram piece of heated copper is dropped into 50.0 grams of room temperature water. Which of the following statements is true? The specific heat of copper is 0.385 J/g°C and the specific heat of water is 4.184 J/g°C.
- The heat lost by the copper is equal in magnitude to the heat absorbed by the water.
- The final temperature of the copper will be equal to the final temperature of the water
- ΔT for the copper = - ΔT for the water
A. i only
B. i and ii only
C. iii only
D. i, ii and iii (all three are true)
E. i and ii only
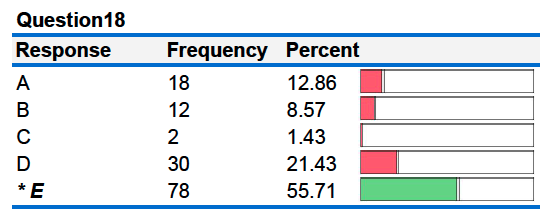
19. Two metal cylinders, X and Y, are heated to the same final temperature. Cylinder X has twice the mass of Cylinder Y. Cylinder X is a different metal compared to cylinder Y.
Cylinder X. Cylinder Y.
Each is plunged into separate insulated containers holding 100 mL of water at 25°C. If the final temperature in both containers is the same, the specific heat capacity of metal X is _________ as the specific heat capacity of metal Y.
A. four times as large D. half as large
B. twice as large E. one fourth as large
C. the same
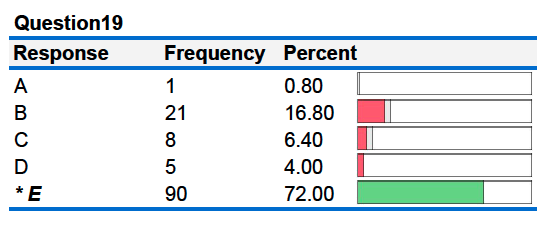
19B. Each is plunged into separate insulated containers holding 100 mL of water at 25°C. If the final temperature in both containers is the same, the specific heat capacity of metal X is _________ as the specific heat capacity of metal Y.
A. four times as large D. one fourth as large
B. twice as large E. half as large
C. the same
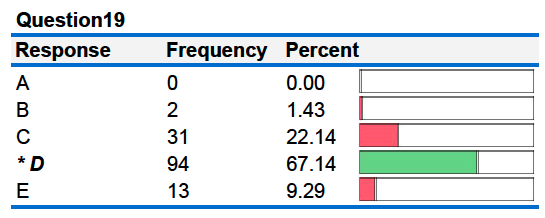
Note: For Question 19, for metal X and metal Y the heat released by the hot metal is equal to the heat gained by the cool water, but the change in temperature of the metal is not the same as the change in temperature of the water. The fact that many students selected "the same" or "all three are true" as a response indicates these students do not distinguish correctly between heat and temperature. These students view heat and temperature as the same thing.
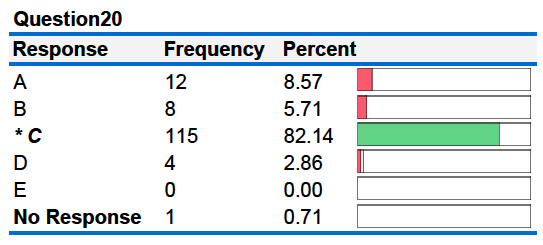
20. When 100. grams of an unknown metal at 80.00°C is placed in a calorimeter containing 100. grams of water, the temperature of the water rises from 20.00°C to 25.00°C. Given that the specific heat of water is 4.184 J/g°C, what is the specific heat of the metal?
A. 0.26 J/g°C D. 1.90 J/g°C
B. 0.38 J/g°C E. 4.18 J/g°C
C. 1.52 J/g°C
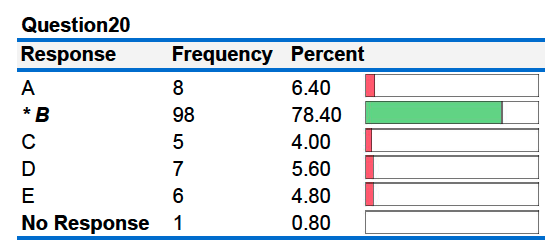
20B. When 100. grams of an unknown metal at 80.00°C is placed in a calorimeter containing 100. grams of water, the temperature of the water rises from 20.00°C to 25.00°C. Given that the specific heat of water is 4.184 J/g°C, what is the specific heat of the metal?
A. 0.26 J/g°C B. 1.90 J/g°C
C. 0.38 J/g°C D. 4.18 J/g°C
E. 1.52 J/g°C