An investigation of the energy produced by an acid-base chemical reaction starts with mixing hydrochloric acid and aqueous sodium hydroxide in a calorimeter. A computer animation is used to model how kinetic energy is transfered among water molecules and ions during the reaction.
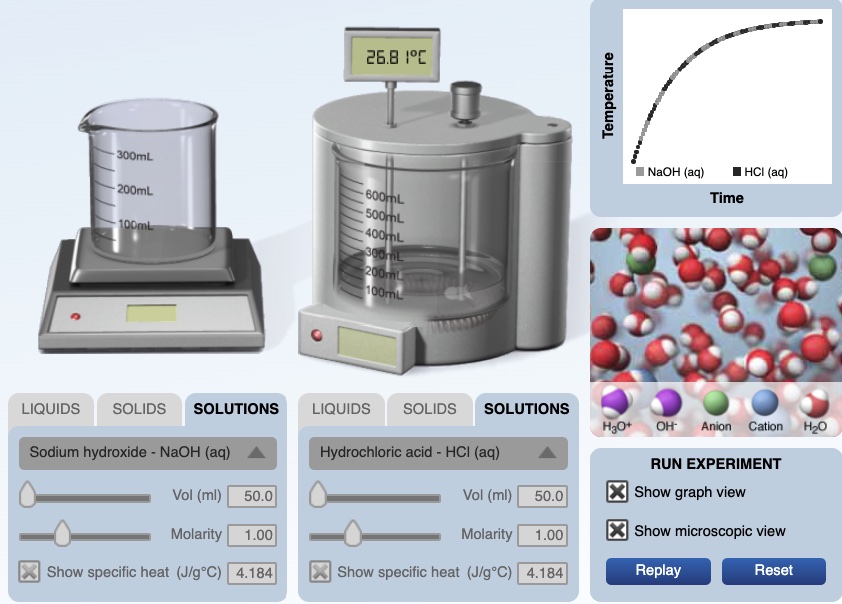
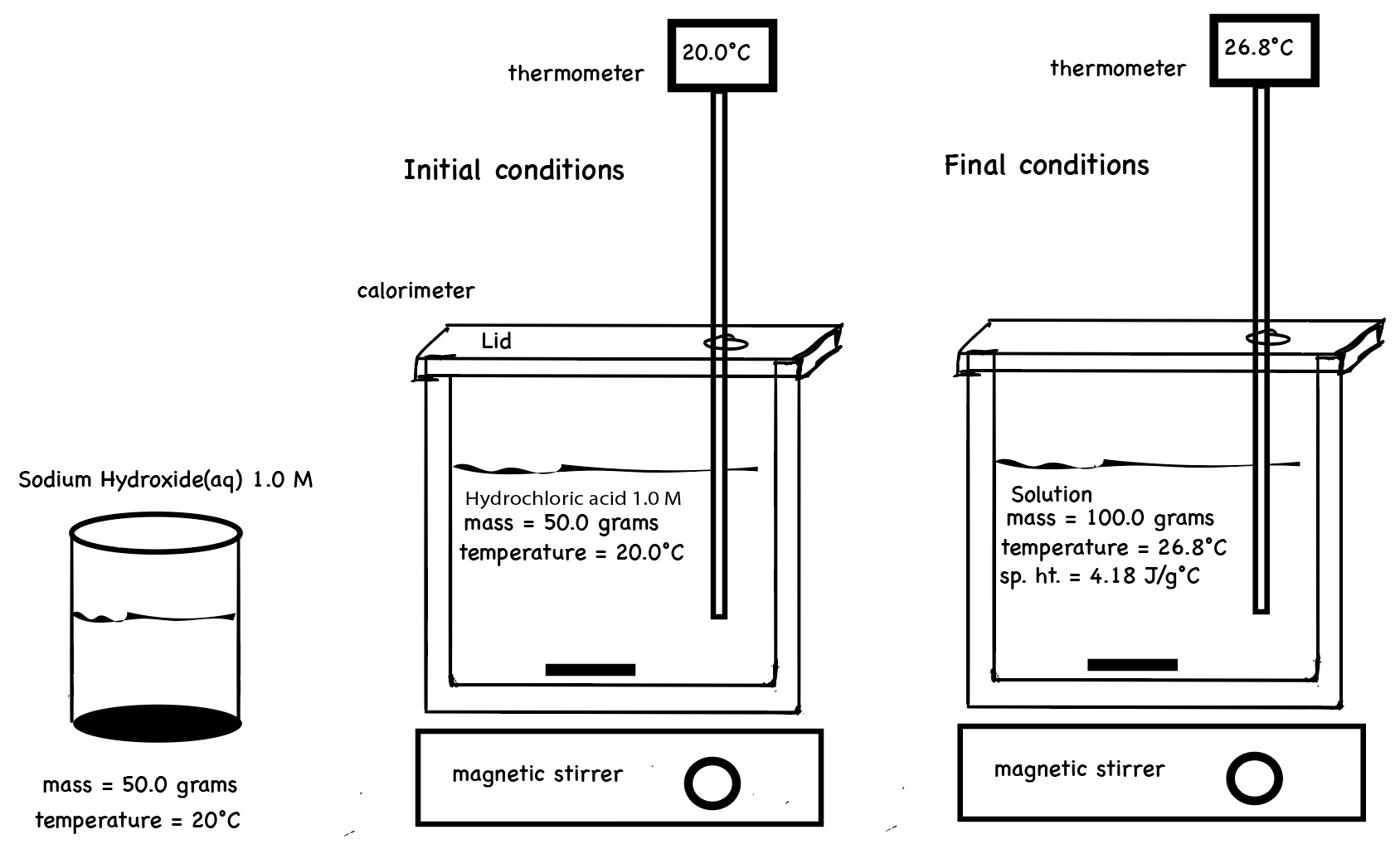
Equal volumes, 50.0 mL, of 3.0 M hydrochloric acid and 3.0 M sodium hydroxide solutions having an initial temperature of 20.0°C react in a calorimeter. The resultant solution records a temperature of 40.0°C. The thermal energy gained by the resultant solution, qsolution ,can be calculated using
qsolution = m c ∆T
where m is the total mass of the resultant solution, c is the specific heat capacity of the resultant solution,
and ∆T is the change in temperature of the solution (T final - T initial)
Since the solutions are mostly water, the solutions are assumed to have a density of 1.00 g/mL and a specific heat of 4.18 J/g°C. The reaction of an aqueous hydrochloric acid solution with an aqueous sodium hydroxide solution is represented by a balanced chemical equation
HCl(aq) + NaOH(aq) -> NaCl(aq) + H2O(l) + Energy

When equal volumes of 3.0 M acid and base solutions at room temperature are mixed in a calorimeter, the temperature of the solution increases.
The logical train of thought is: the temperature of the solution inceased, the solution gained kinetic energy in the form of thermal energy. This energy was released by the chemical reaction as potential energy due to bonds breaking and bonds forming. The reaction of HCl(aq), a strong acid, with NaOH(aq), a strong base, is an exothermic reaction.
This demonstration is usually performed when topics in thermochemistry or thermodynamics are being discussed. The big idea for most calorimetry demonstrations is energy is conserved. Energy cannot be created or destroyed, but it can be exchanged. Remember, heat is not a type or form of energy!
qlost+ qgain = 0 or qreleased + qgain = 0
Thermochemistry
determine the change in enthalpy for the reaction, ∆Hrxn
q = m c ∆T
Since the reaction was conducted under conditions of constant pressure and the reaction has a 1:1 stoichiometric ratio, the change in enthalpy of the reaction, ∆Hrxn, can be determined
∆Hrxn = qrxn / # moles of limiting reactant
Calculating the limiting reactant. Since we have equimolar quantities of acid and base, either can be used in the calculation.
This web page is for chemistry instructors.
Web Page Author: T. Greenbowe, University of Oregon. This page is under construction.
This chemical reaction is classified as an exothermic reaction.
This demonstration also illustrates how the formation of water (one of the driving forces) can act to drive a reaction to spontaneity. This is a neutralization reaction with the hydroxide ion acting as the base and the hydronium ion acting as the
The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is:
HCl(aq) + NaOH(aq) -> NaCl(aq) + H2O(l) + Energy
Net ionic equation: H3O+(aq) + OH-(aq) => 2 H2O(l) + Energy
Since theses are dilute solutions and are mostly water, assume that the densities of the solutions and the specific heat capacities of the solutions are approximately 1.0 g/ml and 4.18 J/g°C, respectively. In order for students to grasp the main concepts associated with this demonstration, assume that the calorimeter is very well insulated and that no energy is lost to the surroundings or walls of the container.
The thermal energy gained by the resultant solution is qsolution. We can measure the mass of the solution, the initial temperature of the solution, and the final temperature of the solution.
qsolution = m c ∆T where m is the total mass of the resultant solution, c is the specific heat capacity of the solution,
and the change in temperature of the solution is ∆T = Tf -Ti
qsolution = (50. g HCl + 50. g NaOH)(4.18 J/g °C)(40.0°C - 20.0 °C) = +8,360 J
The resultant solution gained 8,360 J of energy.
We cannot directly measure anyting about the chemical reaction. A chemical reaction has no mass. A chemical reaction consists of bonds breaking and bonds forming. Chemical bonds have no mass and no temperature.
The thermal energy released by the reaction is qreaction. We can infer what energy was released by the chemical reaction, because we know what energy was gained by the soluiton. By the law of conservation of energy:
qreaction + qsolution = 0 qreaction = -qsolution = -8,360 J
The chemical reaction released 8,360 J of energy.
We need to calculate the change in enthaply for the reaction.
At constant pressure, the enthalpy change for the reaction for the amounts of acid and base that react are
∆H rxn = qreaction / # moles of limiting reactant
The limiting reactant is either the HCl or the NaOH since there are equimolar amounts present
0.050 L HCl x 3.00 mole HCl/L HCl = 0.150 mole HCl
For the amounts of acid and base that react, the enthalpy change for the reaction
∆H rxn = qreaction / # moles of limiting reactant = -8,360 J / 0.150 mole HCl = - 55,730 J/mole HCl or -55.7 kJ/mole HCl or -55.7 kJ/mole of reaction
Materials
- Two Styrofoam coffee cups – nested
- Lid
- Digital Thermometer or a Vernier Temperature Probe or Thermocouple with interface to computer* Logger Pro or Logger Lite software
- PC or Mac lap-top with appropriate software for displaying the temperature
- 50 mL 3.0 M HCl
- 50 mL 3.0 M NaOH
- 2 100 mL graduated cylinders
- ring stand and 2 clamps (see diagram and digital image of the set-up)
Safety Precautions
The 3.0 M HCl solution is corrosive. The 3.0 M NaOH solution is caustic. Both the acid and base solutions can cause burns to exposed skin and damage to eyes. Use gloves and eye protection while preparing and performing the experiments.
One day of lead time is required for this project.
Making this demonstration interactive - active learning
The instructor should "frame" the demonstration and guide the discussion. After students observe the initial conditions of the solutions and observe the results of the demonstration, it is important for the students to be allowed to discuss what gains heat and what loses heat in this chemical process before the instructors tells the students the answers. Students should be asked to identify what gains heat and what looses heat - use a series of Clicker Questions. Ask "What gains thermal energy?" "How much thermal energy is released or gained by the solution?" "How much thermal energy is released or gained by the reaction?" "What are the primary species present in each solution before the reaction?" "What are the species present in the solution after the reaction?" "How is the the thermal energy manifested - what are the water molecules doing before and after the reaction occurs?"
Student difficulties with thermochemistry concepts
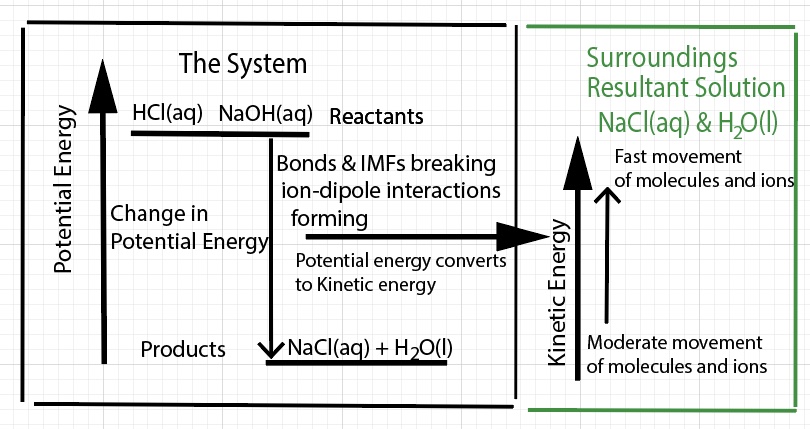
Students have difficulty distinguishing the terms temperature and heat. Students have difficulty with the idea that the bulk material they can see is NOT the chemical reaction. A chemical reaction has no mass, has no specific heat, and does not change temperature. A chemical reaction consists of bonds breaking and bonds forming. Chemical bonds are form of potential energy. In this demonstration, the chemical reaction releases potential energy. The potential energy is converted to kinetic energy. The water molecules and ions in resultant solution receive this increase in kinetic energy. The water molecules and ions move and vibrate vigorously. The water and dissolved chemicals gain heat energy.
The experimental set-up in this calorimetry demonstration seems simple, but it is complex. The chemical reaction occurs within the solution. We can define the system as the chemical reaction. We can define the surroundings as the resultant solution. When thermal energy is transferred into the surroundings, the solution, from the chemical reaction, the solution increases in temperature.
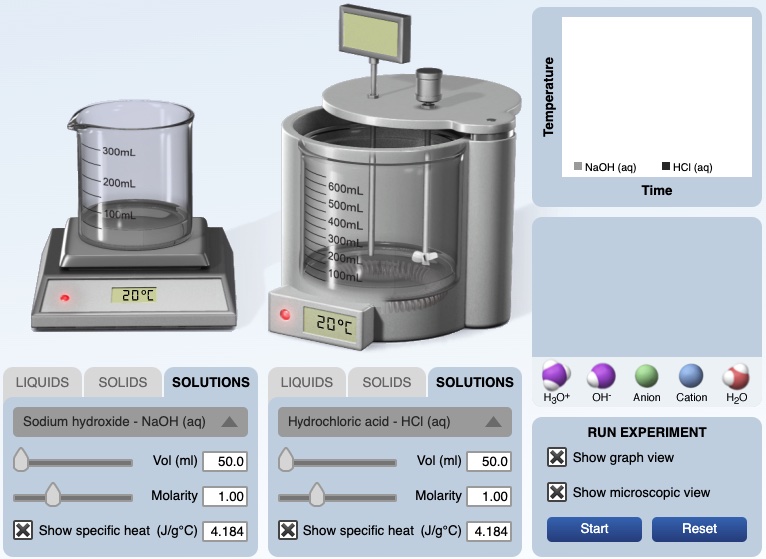
Calorimetry Computer Simulation
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
©2016 Greenbowe, T., Abraham, M., Gelder, J. Calorimetry Computer Simulation. Pearson: Hoboken, NJ.
If you use the simulation in a lesson, cite the simulation. The simulation may not be used in a lesson sold for profit.
The computer simulation has an animation representing the movement of ions and water molecules when HCl(aq) reacts with NaOH(aq) during a chemical reaction. This animation is a model and like all models has limitations. The animation focuses on changes in kinetic energy of particles (molecules and ions) and does not include the contribution of potentail energy, bonds stretching and contracting.
| An animation representing the movement of ions and water molecules when HCl(aq) reacts with NaOH(aq) during a chemical reaction. The water molecules being formed by the reaction have higher kinetic energy compare to the original water molecules in the solution. The newly formed water molecules collide with the original water molecules causing some of the original water molecules to move faster, there is a net increase in kinetic energy of the water molecules. From a previous animation of dissolving salts in water - Water molecules interact with the cations and anions in the solid lattice. The ions in the lattice are vibrating. Water molecules surround the exterior ions and lift the ions from the solid structure. The ions are now hydrated (surrounded by water molecules). These hydration spheres are moving through solution. |
Kinetic energy = (1/2 mv2)average Translational Energy = (3/2) kBT where T is the temperature in units of Kelvin and kB is the Boltzmann constant
Students have a difficult time understanding that through the vibration and movement of atoms and or molecules, as well as a change in potential energy of the system, heat energy is exchanged in the form of potential energy and kinetic energy.
There is a computer animation available depicting the rapid movement of newly formed water molecules as a result of an acid-base reaction to accompany this demonstration.
There is a calorimetry computer simulation available to accompany this demonstration.
There is an in-class POGIL-like activity to accompany this demonstration.
There are a set of interactive guided-inquiry Power Point slides to accompany this demonstration.
Showing acid-base neutralization calorimetry demonstration, the computer animation at the particle level, and the chemical equations helps students connect the macroscopic, microscopic (particle), and symbolic levels of representation - Alex Johnstone's Triangle (Johnstone 1991, 1993)- which leads to a more in-depth understanding of the concepts associated with thermochemistry.
Discussion
Students must have experience working with physical processes involving calorimetry prior to learning about chemical reactions involving calorimetry and thermochemistry. Calorimetry is the process by which the thermal energy exchanged in a chemical or physical process can be determined. The apparatus is the calorimeter. A coffee cup calorimeter made of styrofoam is effective in preventing heat transfer between the system and the environment. Because the solution in the calorimeter (the cup) is open to the atmosphere, as long as the pressure does not change while performing the demonstration, this is constant pressure calorimetry. The thermal energy exchanged by the reaction, qreaction, can be used to determine the change in enthalpy of the reaction.
Thermodynamics
With respect to the thermodynamics associated with a chemical reaction, there are two main factors to consider - a change in enthalpy, ∆H, and a change in entropy, ∆S. For every chemical reaction there is a change in enthalpy. The change in enthalpy of this acid-base reaction overall releases potential energy (an exothermic process). Potential energy is converted (or transformed) to kinetic energy. The water molecules and ions comprising the solution absorb this kinetic energy (an endothermic process). For this chemical reaction there is an increase in entropy - an increase in microstates - due to molecules and ions dispersing throughout a solvent, water, and moving about (disorder).
Conceptual Understanding for an Exothermic Dissolving Process. Ask students: "Where does the energy come from and go to?"
This exothermic chemical reaction releases potential energy. Thermal energy is defined as the total kinetic energy of the atoms and molecules involved in the reaction or process. One indication of what is going on with the particles comes from a measurement of temperature of the solution. When HCl and NaOH react, in a calorimeter,the observation is the temperature of the resultant solution (the surroundings) increases. The inferences are 1) the solution gained thermal energy - the kinetic energy of the water molecules and ions increases, 2) the chemical reaction (the system) released potential energy, 3) the potential energy of the system is lowered. Some of the potential energy is converted to thermal energy, and 4) there is an increase in entropy of the system (more microstates).
"The internal energy of a system (the chemical reaction) is the sum of its kinetic energy and potential energy."
In an exothermic reaction or process, a big contribution of the net energy released is the change in potential energy (released) when bonds and ion-dipole interactions form between cations and anions of the salt and water molecules. A release of potential energy is converted into kinetic energy. The water molecules move faster. These faster moving water molecules other water molecules. In an exothermic dissolving process, there is an excess of kinetic energy of the water molecules. The water molecules and ions hit the thermometer with more forceful impact and the thermometer indicates a higher temperature for the solution.
See N. Tro (2023) Chemistry: A Molecular Approach (6th ed), Chapter 7 Section 7.6 page 287, Pearson: Hoboken, NJ. for a good discussion of exothermic and endothermic processes from the molecular view.
In an exothermic reaction, the value of ∆H is negative, energy is released by the chemical reaction (bonds breaking and bonds forming). Overall, potential energy of the "system" is converted to kinetic energy of the "surrounding", the solution. This increase in kinetic energy goes to increasing the movement of water molecules and ions. There is an increase in entropy (∆S). An increase in entropy is a driving force. The formation of water molecules is also a driving force.
System and Surrounding
A system is a part of the world scientists choose to investigate. For the purpose of this discussion, we assume the calorimeter contains an isolated system as no heat is exchanged outside of the calorimeter. Because the acid and base solutions are mixed together, the identification of system and surrounding can be straight forward. We assume the calorimeter has a lid and is a good insulator. One can include a calorimeter correction factor, the heat capacity of the calorimeter, in the calculation. Since we have an excellent calorimeter, no thermal energy enters or leaves the calorimeter. We assume the inside walls of the calorimeter do not add or subtract thermal energy from the solution. The world outside the calorimeter is not influenced by the experiments. Chemists look at reactions from the system point of view. If energy is released by the reaction, this is a negative value.
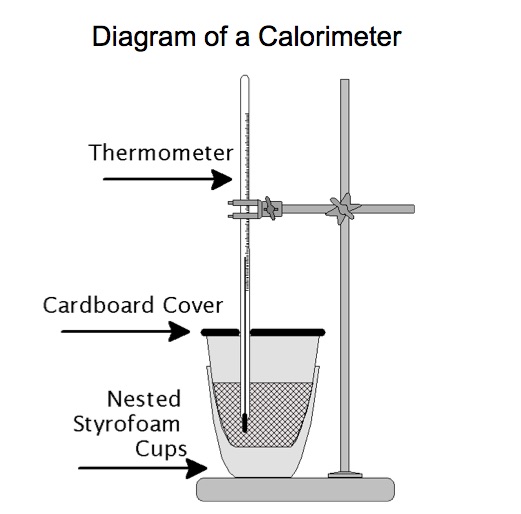
Chemists often define a system as a chemical reaction or a physical process. To be specific, a system can be defined as the reactants and products of a chemical reaction. However, by reactants and products some chemists mean the bond breaking and bond forming process (no mass), not the bulk chemicals (with mass). In the case of an acid-base reaction, we define the system as the "chemical reaction", the change in bonds and intermolecular forces associated with the reactants and products. We cannot directly measure anything about the chemical reaction. We must infer what the reaction did by what happens to the resultant solution. The chemica reaction consists of a series of steps at the particle level involving breaking bonds and interactions and forming bonds and interactions. Bonds, intermolecular forces, and interactions do not have mass nor do these entities have a temperature that can be measured.
Chemists often define the surroundings as the calorimeter, the experimenter, the room in which the experiment occurs, and everything beyond the room. In the case of reaction HCl(aq) and NaOH(aq) in a calorimeter with an isolated system, surroundings are defined as the resultant solution. The solution has a total mass. The solution consists of the mass of water and the mass of the chemicals - in this case HCl and NaOH - hydronium ions, H3O+, chloride ions, Cl-, and Na+ ions. One cannot ignore the mass of the ions. The solution has an initial and final temperature, which can be measured. The change in temperature of the solution can be calculated. During the reaction, the water, the solvent, increases in temperature and due to thermal equilibrium, the bulk chemicals, which are the solute, in the solution also increase in temperature. The solution has a specific heat capacity. The heat energy absorbed or released by the solution can be calculated using the total mass of the solution, the specific heat capacity of the solution and the change in temperature of the solution.
In the case of reaction HCl(aq) with NaOH(aq), the resultant NaCl solution increased in temperature. A thermometer measures the temperature of the solution which consists mostly of water molecules and some cations and anions. We infer that the NaCl(aq) solution gained thermal energy (an endothermic process) and that the chemical reaction released this thermal energy (an exothermic process).
A chemical reaction involves bond breaking, disruption of hydrogen bonding, and formation of ion-dipole interactions. Chemical bonds, intermolecular forces, and interactions have no mass and have no association with temperature. Breaking and forming chemical bonds involves changing the potential energy of the species involved in chemical reaction(water molecules and ions). A chemical equation has no mass. A chemical reaction has no mass and no temperature. One cannot directly see a chemical reaction - with respect seeing bonds breaking and bonds forming. We measure the mass, initial and final temperature of the solution. The bulk chemicals and the water - are not the reaction. If one observes the mixing of acid and bases solutions there is no visual clues that a reaction occurs. Individuals cannot see the actual "reaction". If a reaction releases heat energy, the resultant solution gains this heat energy. We calculate the thermal energy absorbed by the reaction, q reaction based on what the solution did. The thermal energy absorbed by the solution is a positive quantity.
For this chemical reaction, one observes an increase in temperature of the resultant solution. The inference is the solution gained thermal energy. The Law of Conservation of Energy states energy cannot be created or destroyed. Remember, heat is not a type (or form) of energy. We will attempt to avoid using the term heat.
In every calorimetry experiment something releases thermal energy and something gains this thermal energy. This is the big idea for calorimetry and must be emphasized to students multiple times. The chemical reaction releases thermal energy and the solution absorbs the thermal energy. If we calculate the thermal energy absorbed by a solution, q solution, we infer the thermal energy released by a chemical reaction, q reaction, is equal but opposite in sign. This is an application of the Law of Conservation of Energy which is part of the First Law of Thermodynamics. The following equation can be characterizes as an application of the Law of Conservation of Energy - it is not THE Law of Conservation of Energy
q solution + q reaction = 0
Identify What Releases Thermal Energy and What Gains Thermal Energy?
It is better to have students first identify what releases thermal energy and what gains thermal energy. After students do an analysis of the experiment, then identify a system and surrounding. When hydrochloric acid reacts with aqueous sodium hydroxide in a calorimeter the chemical reaction releases thermal energy and the resultant solution gains thermal energy.
Energy is transferred during a chemical reaction from several different sources. There is a net change in enthalpy, ∆H, of the chemical reaction.
∆Hreaction = H reactants - H products
For a process that releases energy, the enthalpy of the products are lower compared to the enthalpy of the reactants. The reactants have stronger bonds compared to the products. In the case of reacting HCl and NaOH, the HCl and NaOH interactions are stronger compared to the NaCl(aq) interactions.
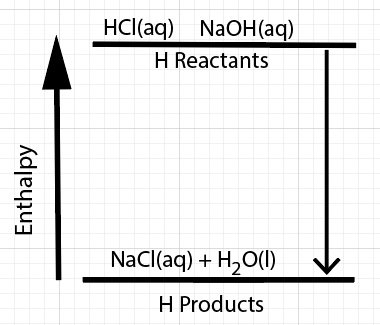
Overall, reacting HCl and NaOH is an exothermic process. By the process we mean bonds breaking and forming and interactions breaking and forming. The heat of neutralization, change in enthalpy of reaction, ∆Hneutralization, is negative.
Physics
With respect to the physics associated with a chemical reaction there are two main factors to consider - changes in potential energy and changes in kinetic energy. For an exothermic reaction there is a net lowering of the potential energy of the "system - defined as bonds and interactions" and there is an overall increase in the kinetic energy of particles (molecules and ions) in the resultant solution. Bond breaking, bond forming, and ion-dipole interactions forming and breaking are changes in potential energy. The potential energy released by the chemical reaction is transformed (or converted) to an increase in kinetic energy of the molecules and ions in the solution. How potential energy is transformed to kinetic energy is difficult to represent. Reacting HCl and NaOH in water results in a net release of potential energy by the chemical bonds and ion-dipole interactions. Overall, potential energy is converted (or transformed into) to kinetic energy of vibrating atoms when bonds break. In the case of forming aqueous sodium chloride the ions and water molecules are vibrating moderately and moving about. With this HCl NaOH reaction, the decrease potential energy is transformed to an increase in kinetic energy of the molecules and ions.
Learning Objectives
After observing the demonstration and doing the in-class activities, students should be able to
1. Identify what is releasing thermal energy and what is gaining thermal for a given calorimetry experiment.
2. Identify the system and the surroundings for a given calorimetry experiment.
3. Calculate the thermal energy gained or released by a solution, qsolution, involved in a given calorimetry experiment: total mass of the solution, specific heat of the solution, change in temperature of the solution: qsolution = m c ∆T
4. Apply the law of conservation of energy to calorimeter experiments, qreaction + qsolution= 0
5. If the calorimetry experiment is carried out under constant pressure conditions, calculate ∆H for the reaction.
6. Given the sign of the ∆Hrxn, classify the reaction as being endothermic or exothermic. Note: Given the change in temperature O(or temperature measurement) of a solution one can identify if the solution gained thermal energy - an endothermic process - or released thermal energy - an exothermic process. From here one can then infer what the reaction did.
7. Given the change in enthalpy for a reaction, the amounts of reactants, and a balanced chemical equation, calculate the heat exchanged for a reaction.
References
Cooper, M.M., Klymkowsky, M.W. (2013). The Trouble with Chemical Energy: Why Understanding Bond Energies Requires an Interdisciplinary Systems Approach. CBE—Life Sciences Education, Vol. 12, 306–312, Summer 2013.
Greenbowe, T.J. and Meltzer, D.E. (2003). “Student Learning of Thermochemical Concepts in the Context of Solution Calorimetry.” International Journal of Science Education, 25(7), 779-800.
J. Kotz, P. Treichel, J. Townsend ( 2009) Chemistry & Chemical Reactivity 7th ed. Instructors Edition; Brooks/Cole.
Gelder, John I.; Abraham, M.R., Greenbowe, T.J. (2017). The Next Generation Project: Computer Simulations, Scenarios, Animations - Calorimetry. Pearson Education, Inc.: Upper Saddle River, NJ. http://dbpoc.com/pearson/chemsims/gold/calorgold/Calor.php
Russell, JW, Kozma, RB, Jones, J Wykoff, N Marx, J Davis (1997). Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts Journal of Chemical Education, 74 (3), 330.
Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Learning, 7: 75–83.
Johnstone, A. H. (1982). Macro- and micro-chemistry. School Science Review, 64, 377–379.
