I. Calorimetry Computer SimulationThe calorimeter computer simulation is designed to determine the thermal energy transferred, q, and the change in enthalpy of solution,
∆H solution, (or commonly called the heat of dissolution, ∆Hdissolving process ) of various soluble solid ionic salts dissolved in water.
- Instructors and students can vary the mass of water and the mass of the solid.
- Record the temperature of the water and salt before adding the solid to the water.
- Record the temperature of the resultant solution after dissolving the solid.
- Identify what gains thermal energy and what releases thermal energy.
- Calculate the thermal energy, q solution, absorbed or released by the resultant solution.
- Invoke an application of the law of conservation of energy q loss + q gain = 0 to transition from the thermal energy exchanged by the resultant solution to the thermal energy released or absorbed by the dissolving process represented by electrostatic bonds being broken in the lattice and ion-dipole intermolecular forces (IMFs) being formed.
- Calculate the thermal energy, q dissolving process, absorbed or released by the dissolving process.
- q dissolving process + q solution = 0
- Calculate the change in enthalpy associated with the dissolving process: ∆H solution = ∆Hdissolving process
- Use the animation, explain how thermal energy is transferred at the particle level.
For example, the following panels are screen shots of the calorimertry simulation, initial and final stages. 14.0 grams of lithium chloride at 20.00°C are added to 100.0 grams of water at 20.00°C. The solution is stirred and a final temperatrue of of the resultant solution, 43.82°C is recorded. The specific heat of the solution is 4.184 J/g°C.
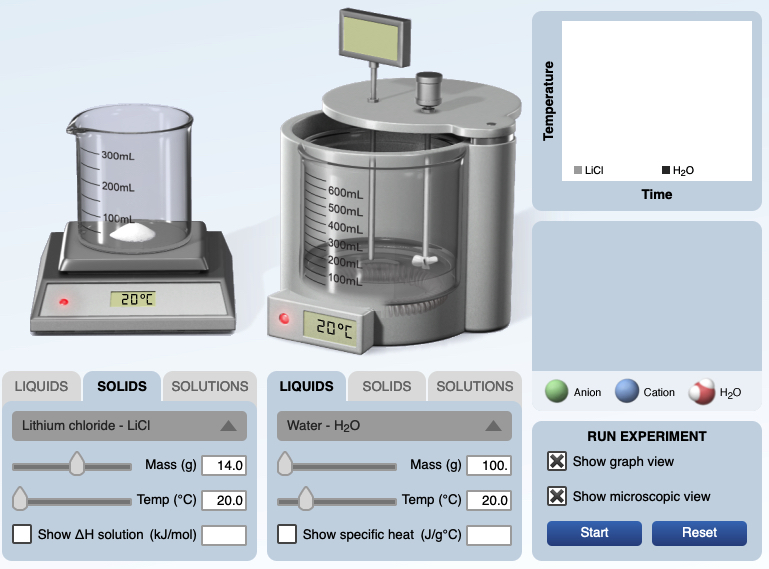 | 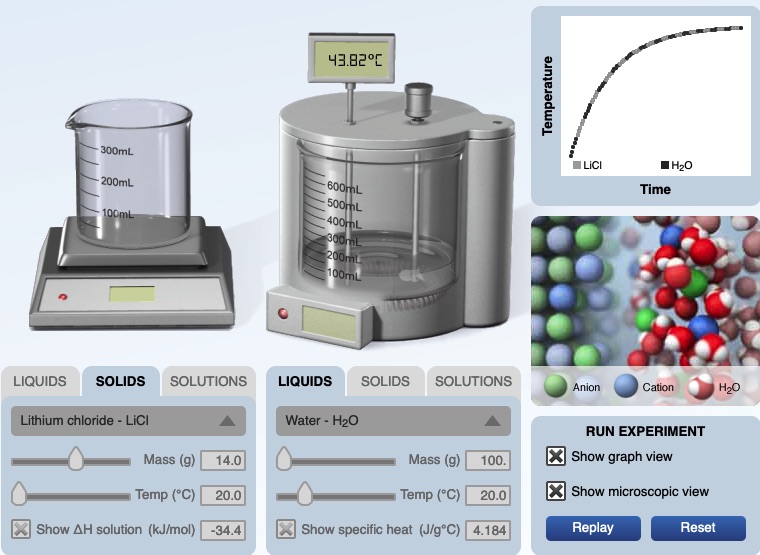 |
The HTML5 calorimetry computer simulation is available at the following URL.
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
If you use the simulation, please cite the simulation. Calorimeter Simulation ©2016 Greenbowe, T., Abraham, M., Gelder, J. Pearson: Hoboken, NJ.
Web page author: T. Greenbowe, University of Oregon. This page is under construction.
Currently, Firefox 111.0.1 is doing the best at playing the embedded animations (April, 2023).
The following is an image from the computer simulation experiment for dissolving lithium chloride in water. Before mixing, the temperature of both the solid lithium chloride and water is 20.00°C. After dissolving the temperature of the solution is 43.82°C. A graph of temperature vs. time shows an increase in temperature of the solution.

The following two diagrams attempt to illustrate the initial conditions of a calorimetry experiment - dissolving solid lithium chloride in water. Both the solid and the water start at room temperature. The solid salt is added, the water is stirred, the salt dissolves. The resultant solution has a final temperature of 43.8°C.
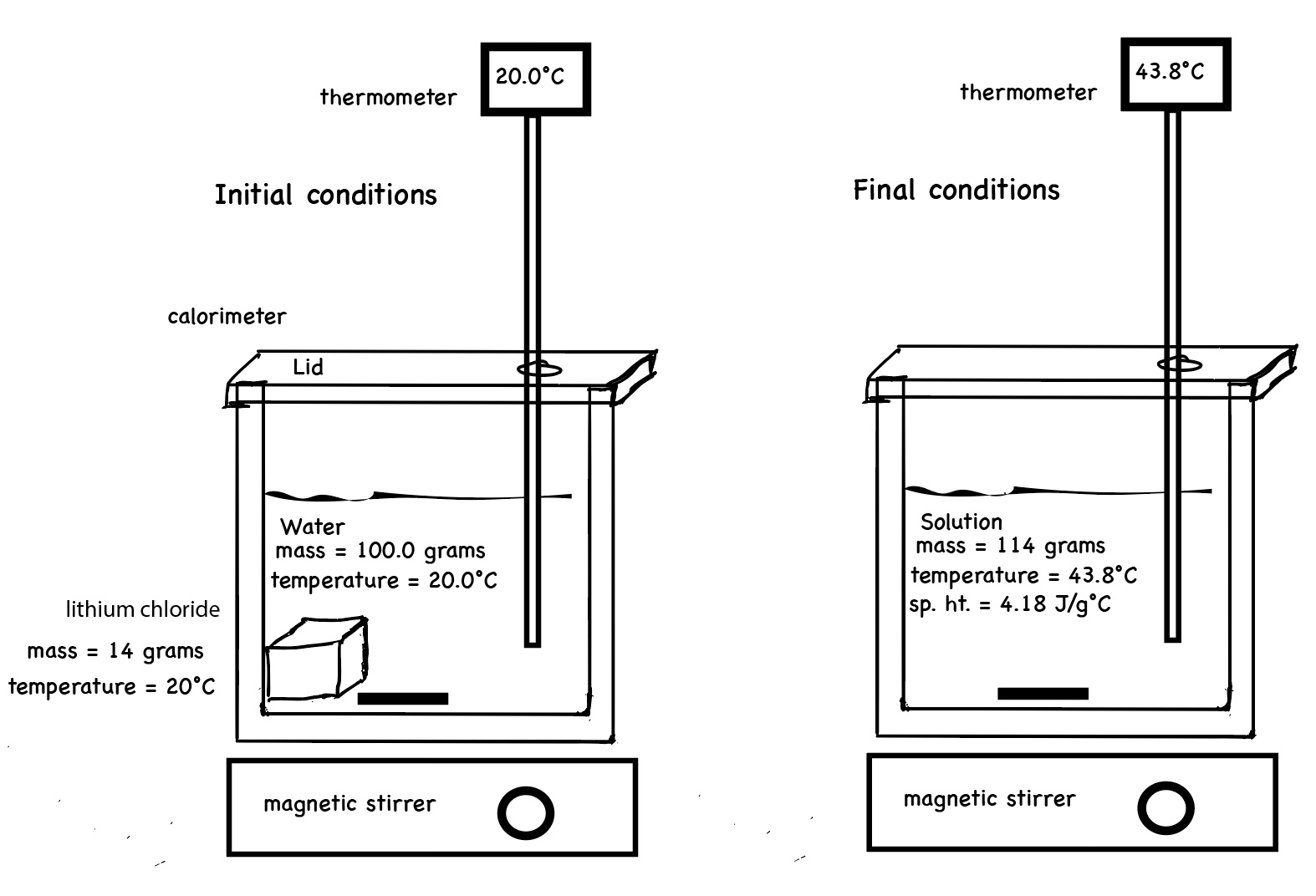
Calculations of ∆H solution for dissolving lithium chloride in water using the data from the simulation of mixing LiCl(s) in water.
The total mass of the solution is 114.0 grams. (100.0 grams of water + 14.0 grams of LiCl)
The change in temperature of the solution is T final - T initial = 43.82°C - 20.00°C = 23.82°C
The moles of LiCl dissolved mass LiCl/Molar Mass LiCl = 14.0 grams LiCl/42.39 g/mol = 0.330 moles LiCl
q solution = m c ∆T = 114.0 g x 4.184 J/g°C x 23.82°C = +11,350 J
q released by the dissolving process + q gained by the solution = 0
q gained by the solution = - q released by the dissolving process = -11,350 J
for a 1:1 stoichiometric ratio
∆Hdissolving process = qdissolving process /moles of limiting reagent
= -11,350 J/0.330 moles LiCl = -34.3 kJ/mol LiCl
∆H solution can be represented as ∆Hdissolving process
∆H solution = -34.3 kJ/mol LiCl. This is an experimental value for ∆H solution.
The dissolving process can be represented by a balanced chemical equation.
The literature value for ∆H solution is -37 kJ/mol.
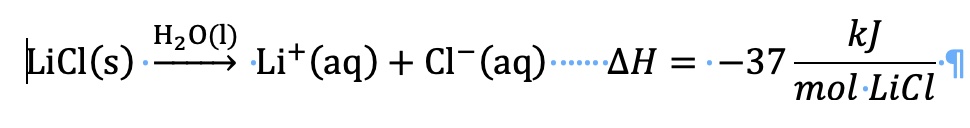
The computer simulation has a particle view (molecular scene) animation of the ions, atoms and or molecules interacting during the dissolving process. This provides a model for thermal energy transfer. This provides an opportunity for students to view a representation of how thermal energy is exchanged during a physical process. For example, when solid lithium chloride is placed in water initially the lithium cations and chloride anions in the LiCl lattice vibrate moderately and the water molecules move slowly.
The following animation provides a short segment of the animation available on the Calorimeter Simulation. The animation is a model, a visual representation, of the movement and vibrations of water molecules and ions during a dissolving process. Dissolving a salt in water is a complex process and this model does not fully capture all of the aspect of the dissolving process.
When the water molecules collide with the surface cations and anions the water molecules orient with the partial negative charge towards the lithium cations, Li+. An ion-dipole interaction forms, releasing energy. Water molecules also orient with the partial positive charge on hydrogen atoms towards the chloride anions, Cl-. An ion-dipole interaction forms, releasing energy. It takes an input of energy to separate a lithium, Li+, cation from a chloride, Cl-, anion in the solid lattice. The water molecules with the newly formed ion-dipole IMF move faster. The temperature of the solution increases due to the faster movement of the water molecules (and associated ions) striking the thermometer. This process continues until all of the solid salt dissolves. This animation representing a dynamic particle interaction helps students to visualize energy transfer at the particle level. This is consistent with the recommendations of Alex Johnstone (1993) and other chemistry educators. Remember this visualization is model and it has limitations.
A. Dissolving lithium chloride in water
We recommend starting the lesson with a chemistry lecture demonstration of dissolving lithium chloride, LiCl, in water and dissolving potassium chloride, KCl, in water. See the link for an example of how an instructor might present this demonstration. At the end of the demonstration, thermometers are used to measure the temperature of the resultant solutions. The demonstration, "heats of reactions: a chemical hot and cold pack demonstration" is presented by a chemistry instructor at Pima Community College, Tucson, Arizona (2020).
https://www.youtube.com/watch?v=CKNG7kHO488 [accessed April, 2023]
The instructor from Pima Coummunity College choose to present a didactic lecture demonstration. We make no judgements as to how instructors choose to present demonstrations. There are other ways an instructor can present the demonstration if they want to include active learning with a guided-inquiry approach. We hope our approach sparks your interest.We recommend the demonstration be paired with the "Calorimeter Simulation".
Solid lithium chloride dissolves in water producing a solution containing a 1:1 ratio of hydrated lithium cations and chloride anions. For example, if 0.050 mole of LiCl dissolves it will generate 0.050 mole of hydrated Li+ ions and 0.050 mole of hydrated Cl- ions, equal number of cations and anions. We can represent this dissolving process by a chemical equation. Dissolving an ionic salt in water is a complex process. For the purpose of this presentation, we will assume that the dissolving process has aspects of both a chemical process (intermolecular forces are broken, ion-ion electrostatic interactions are broken, interactions form, new chemical species formed, in some cases there is a change in pH, and energy is released or absorbed) and a physical process (evaporating the solution leads to recovery of the original salt).
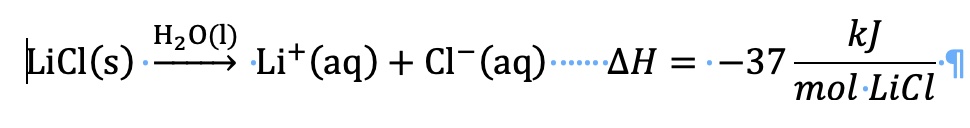
∆H is the change in enthalpy of a process or a reaction. For this dissolving process a subscript can be added to identify the energy change as the change in enthalpy of solution
∆H solution . Commonly called the "heat of solution". In order to avoid confusion with the component, q solution, which is also commonly called the "heat of solution", one should use "change in enthalpy of dissolution, ∆H dissolution .
For this LiCl dissolving process, one observes an increase in temperature of the solution. An increase in temperature of the solution indicates an increase in average molecular speed of the water molecules. When there is an increase in speed of the water molecules, one can infer that the solution gained thermal energy. The Law of Conservation of Energy states energy can not be created or destroyed. The dissolving process (bonds breaking and ion-dipole bonds forming) decreases potential energy. The decrease in potential energy is converted to kinetic energy. The dissolving process releases potential energy and the resultant solution absorbs this energy as kinetic energy, in terms of thermal energy.
A caution about using the term "Heat". Heat is not a form or type of energy. Often the term "heat energy transfer" is used. Thermal energy transfer is a form of energy.
Enthalpy
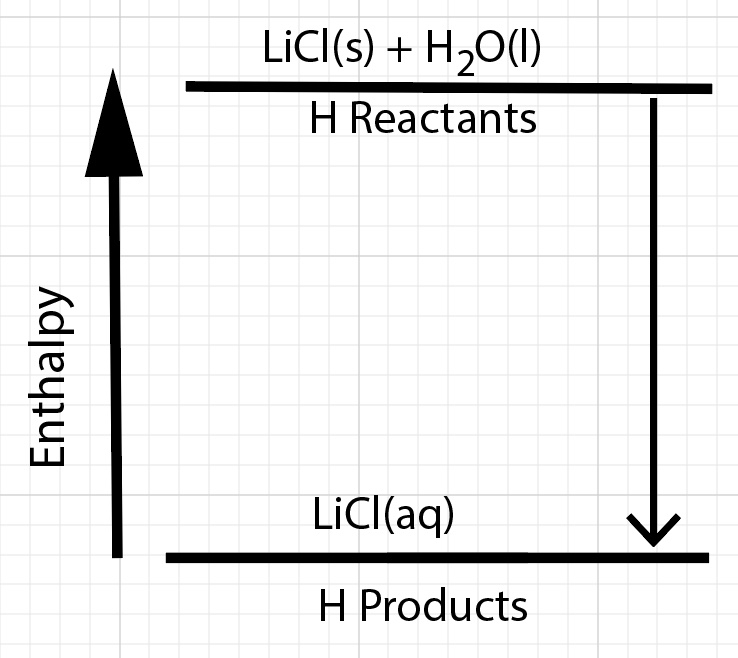
For dissolving lithium chloride in water the products are lower in enthalpy compared to the reactants. We can sketch a qualitative enthalpy digram.
Physics
Energy is transferred during a dissolving process. As a result of the dissolution process, there is a decrease in potential energy. The potential energy released is converted to kinetic energy. With respect to the physics associated with the dissolving process, there are two main factors to consider - changes in potential energy and changes in kinetic energy. For an exothermic dissolving process there is a net lowering of the potential energy of the "system - defined as bonds and interactions" and there is an overall increase in the kinetic energy of particles (molecules and ions) in the resultant solution. Overall, potential energy is converted (or transformed into) to kinetic energy of vibrating atoms when bonds break. In the case of lithium chloride solid, the ions are vibrating moderately in the solid. When ion-ion forces break, the decrease in potential energy is transformed to an increase in kinetic energy of the ions - the ions vibrate vigorously. We can sketch a potentail energy-kinetic energy diagram.
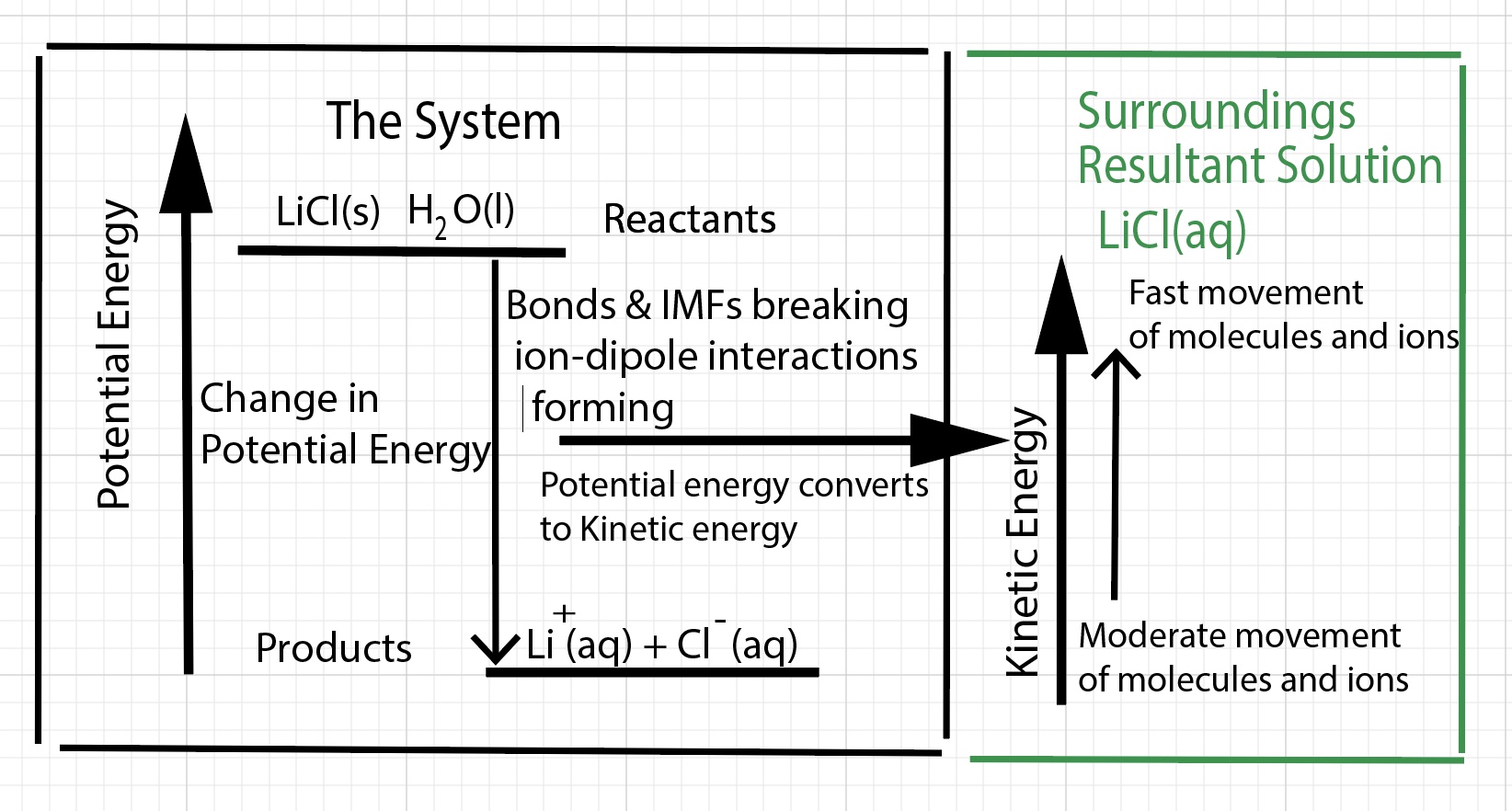
There is a change in enthalpy of the solution. The products are lower in enthalpy compared to the reactants. Dissolving lithium chloride in water results in a net exothermic process.
A theoretical model can represent the dissolving process at the particle level. The solvent molecules, water molecules, need to separate. The energy input required to break hydrogen bonds between water molecules is ∆H solution . The cations and the anions in the crystla lattice need to separate. The energy input required to separate the ions is ∆H solute . This is also the lattice energy. When cations and anions mix with water molecules to form a solution, this is called ∆H mix . In theory, we can determine ∆H solution .
∆H solution = ∆H solute + ∆H solvent + ∆H mix
We can sketch a particle diagram to represent the theoretical steps in any dissolving process.
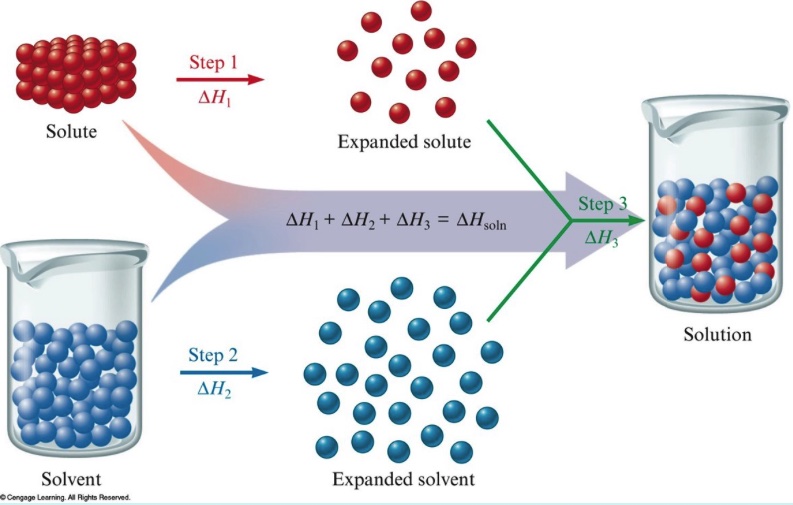
When some salts dissolve, such a LiCl, there is an increase in the temperature of the resultant solution. When other salts dissolve there is a decrease in temperature of the resultant solution. An enthalpy diagram can represent a dissolving process and the sum of contributions of ∆H solute + ∆H solvent + ∆H mix will indicate if a dissolving process is exothermic or endothermic.
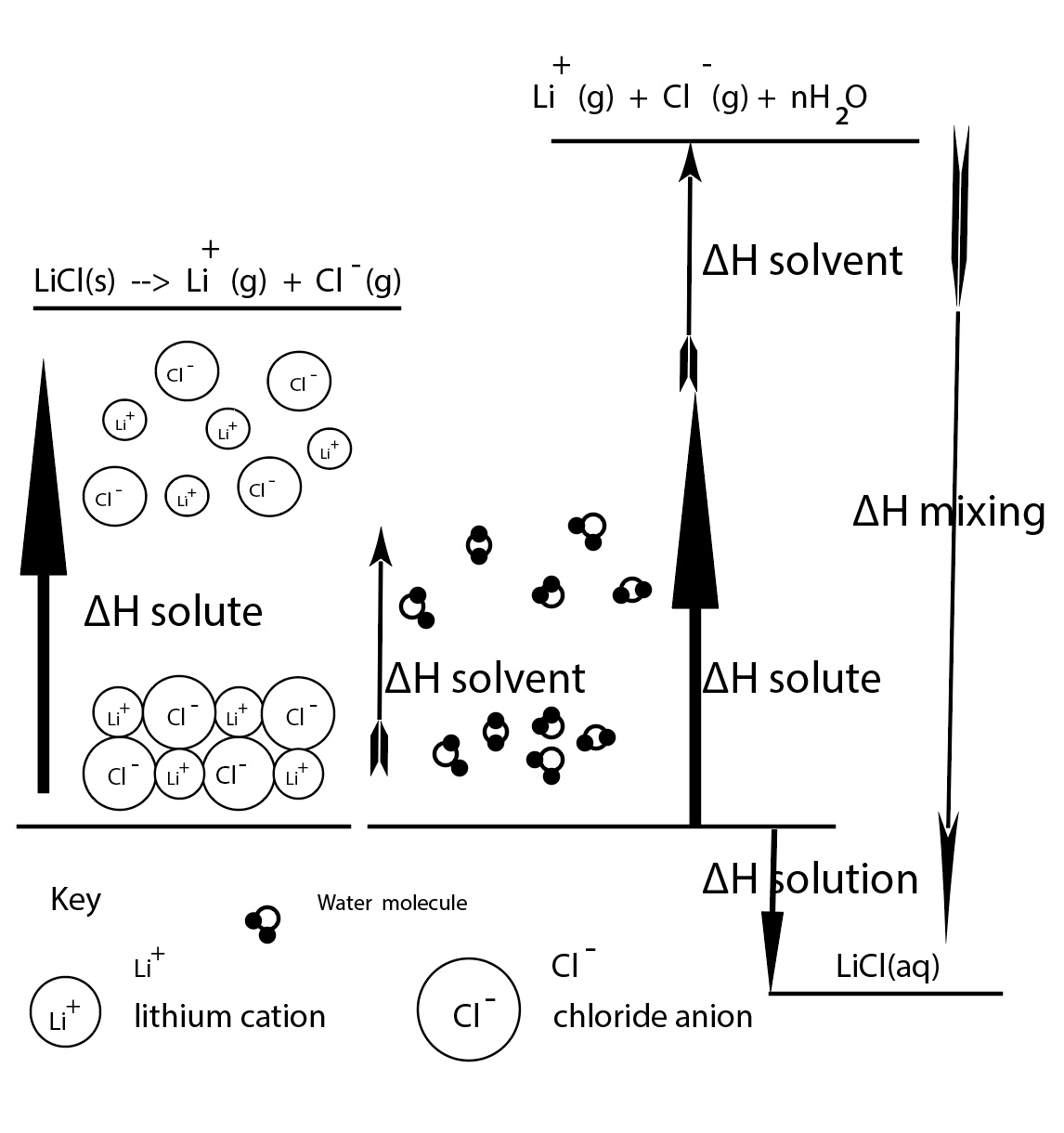
Dissolving a ssolid salt in water does not force the cations and anions into the gas phase. It is difficult to measure ∆H solvent + ∆H mix individually.
In practice, a slight adjustment is made. We can combine ∆H solvent and ∆H mix and this will equal ∆H hydration.
∆H hydration = ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H hydration or ∆H solution = ∆H solute + ∆H hydration
O. Enthalpy Diagram Representing the Energy Involved in the Dissolving Process for LiCl and NH4Cl: A model
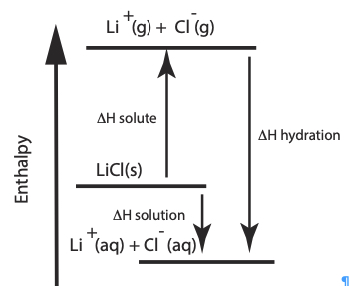 | 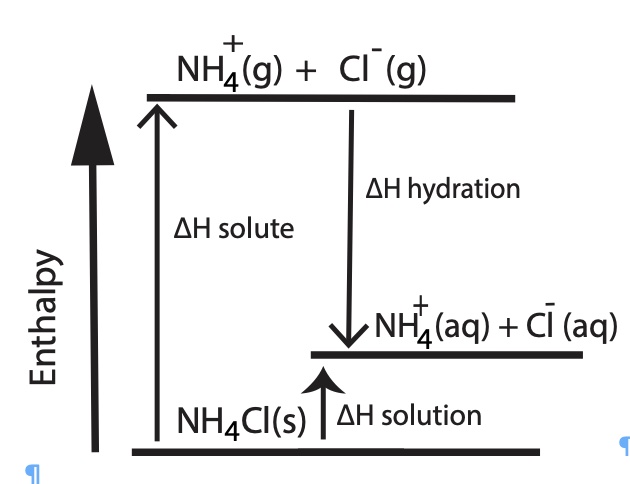 |
The above two enthalpy diagrams help students visualize how it is possible for a salt to have either an overall endothermic dissolving process, change in enthalpy of solution, ∆Hsolution or an overall exothermic process. Lithium chloride has an overall exothermic dissolving process. The heat of hydration of LiCl is greater than the lattice energy, this dissolving process is exothermic. Ammonium chloride has an overall endothermic dissolving process. The heat of hydration is less than the heat of solute (lattice energy), the dissolving process, the heat of solution, is endothermic. One driving force for dissolving a salt is the formation of aqueous species. One other driving force is an increase in entropy.
Calculation of ∆H solution for dissolving lithium chloride in water using ∆H lattice and ∆H hydration values.
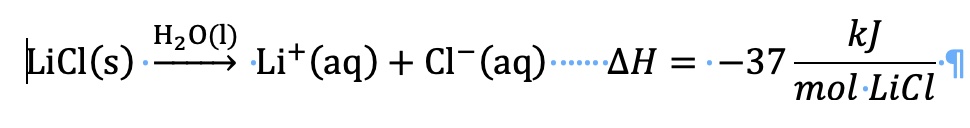
The lattice energy is defined as the energy required to separate one mole of LiCl(s) into gas phase ions. For this calculation, the lattice energy is a positive value. An input of energy is required to separate cations from anions.
∆H solution = ∆H lattice + ∆H hydration
∆H solution = +853 kJ/mol + (-883 kJ/mol) = -30. kJ/mol LiCl
Compare the heat of solution using two different methods.
Using values for ∆H lattice and ∆H hydration, ∆H solution = -30. kJ/mol LiCl
Using measurements and calculation from a calorimeter experiment
∆H dissolving process = ∆H solution = -34.3 kJ/mol LiCl
The literature value for ∆H solution for dissolving LiCl in water is -37.03 kJ/mol LiCl
The heat of solution, change in enthalpy of solution, ∆Hsolution, is negative. Technically, this is often called the change in enthalpy of dissolution or the heat of dissolution.
The calorimeter computer simulation has an animation of a particle view (molecular scene) of the interaction of water molecules with the ions in the solid during the dissolving process. This particle visualization of the dissolving process is a model of energy transfer. As with any model it has limitations and does not capture all of the complexities of the dissolving process.
Conceptual Understanding. Ask students: "Where does the energy come from?"
An animation illustrating energy transfer at the particle level of representation for an exothermic dissolving process. This is a short segment from the simulation. Initially, the cations and anions in the solid are vibrating. The cations and anions are held together by electrostatic forces of attractions. An input of energy is required to separate unlike charged particles. Initially, the water molecules are moving about with moderate movement. When water molecules collide with the cations and anions on the surface of the solid, ion-dipole intermolecular forces form (energy is released). The ions become hydrated. Some of this energy goes into separating cations from anions. Some of the energy goes into separating water molecules. Some of the released energy is absorbed by the water molecules and ions. The water molecules and ions have more kinetic energy and move faster. The water molecules and ions hit the thermometer with more impact and the thermometer indicates a higher temperature for the solution. When LiCl dissolves in water, net energy is released during the dissolving process. <= Currently, Firefox 111.0.1 is doing the best at playing the embedded animations (April, 2023). |
Conceptual Understanding. Ask students: "Where does the energy come from?"
Dissolving ammonium chloride in water.
Solid ammonium chloride dissolves in water producing a solution containing a 1:1 ratio of hydrated ammonium cations and hydrated chloride anions. For example, if 0.050 mole of ammonium chloride dissolves, 0.050 mole of hydrated NH4+ ions form and 0.050 mole of hydrated Cl- ions form, equal number of cations and anions. We can represent this process by a chemical equation.
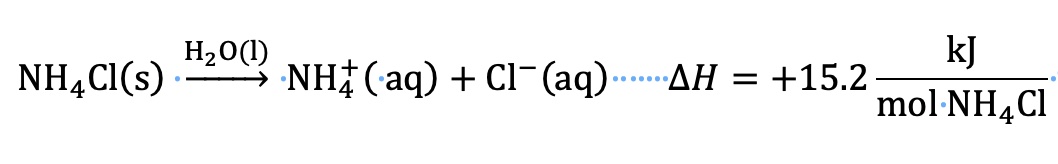
Dissolving an ionic salt in water is a complex process. For the purpose of this presentation, we will assume that the dissolving process has aspects of both a chemical process (new chemical species are formed, and new intermolecular forces are formed) and a physical process (evaporating the solution leads to recovery of the original salt).
For this dissolving process, one observes a decrease in temperature of the solution. The inference is the solution absorbed energy. The Law of Conservation of Energy states energy can not be created or destroyed. The dissolving process absorbed the thermal energy that the solution released. This thermal energy is converted to potential energy. Energy is transferred during a dissolving process. As a result of the dissolution process, there is a change in enthalpy of the solution. The Products have higher enthalpy compared to the reactants. Dissolving ammonium chloride in water results in a net endothermic process.
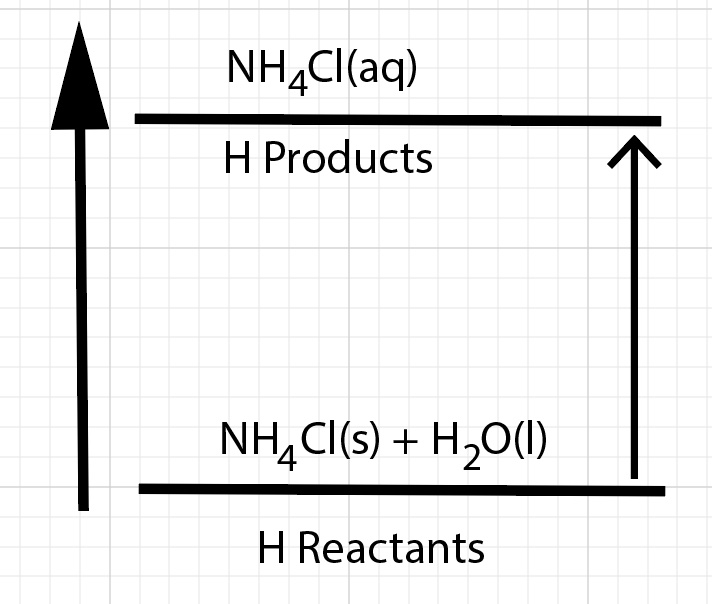
The heat of solution, change in enthalpy of solution, ∆Hsolution, is positive. Technically, this is often called the change in enthalpy of dissolution or the heat of dissolution.
Computer simulation experiment for dissolving ammonium nitrate in water is available. The solid ammonium nitrate and water have an initial temperature of 20.00°C. After dissolving the temperature of the solution is 10.26°C. A temperature versus time graph shows a decrease in temperature of the solution over time.
 | 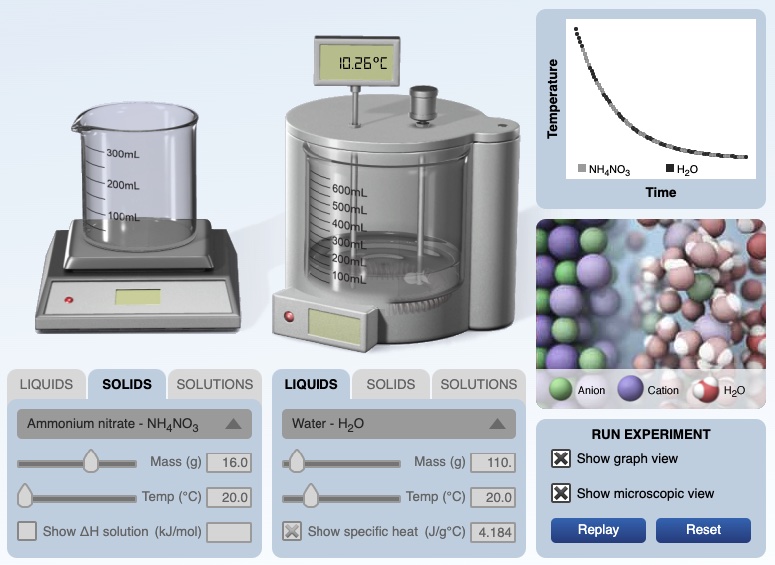 |
The HTML5 calorimetry computer simulation is available at the following URL. https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
If you use the simulation, please cite the simulation. ©2016 Calorimeter Simulation. Greenbowe, T., Abraham, M. Gelder. J., Pearson: Hoboken, NJ. The simulation may not be used in any lesson sold for profit.
The calorimeter computer simulation has an animation of a particle view (molecular scene) of the interaction of water molecules with the ions in the solid during the dissolving process. This particle visualization of the dissolving process is a model of energy transfer. As with any model it has limitations and does not capture all of the complexities of the dissolving process.
As a result of the dissolution process, there is a increase in potential energy. Kinetic energy is converted to potential energy.
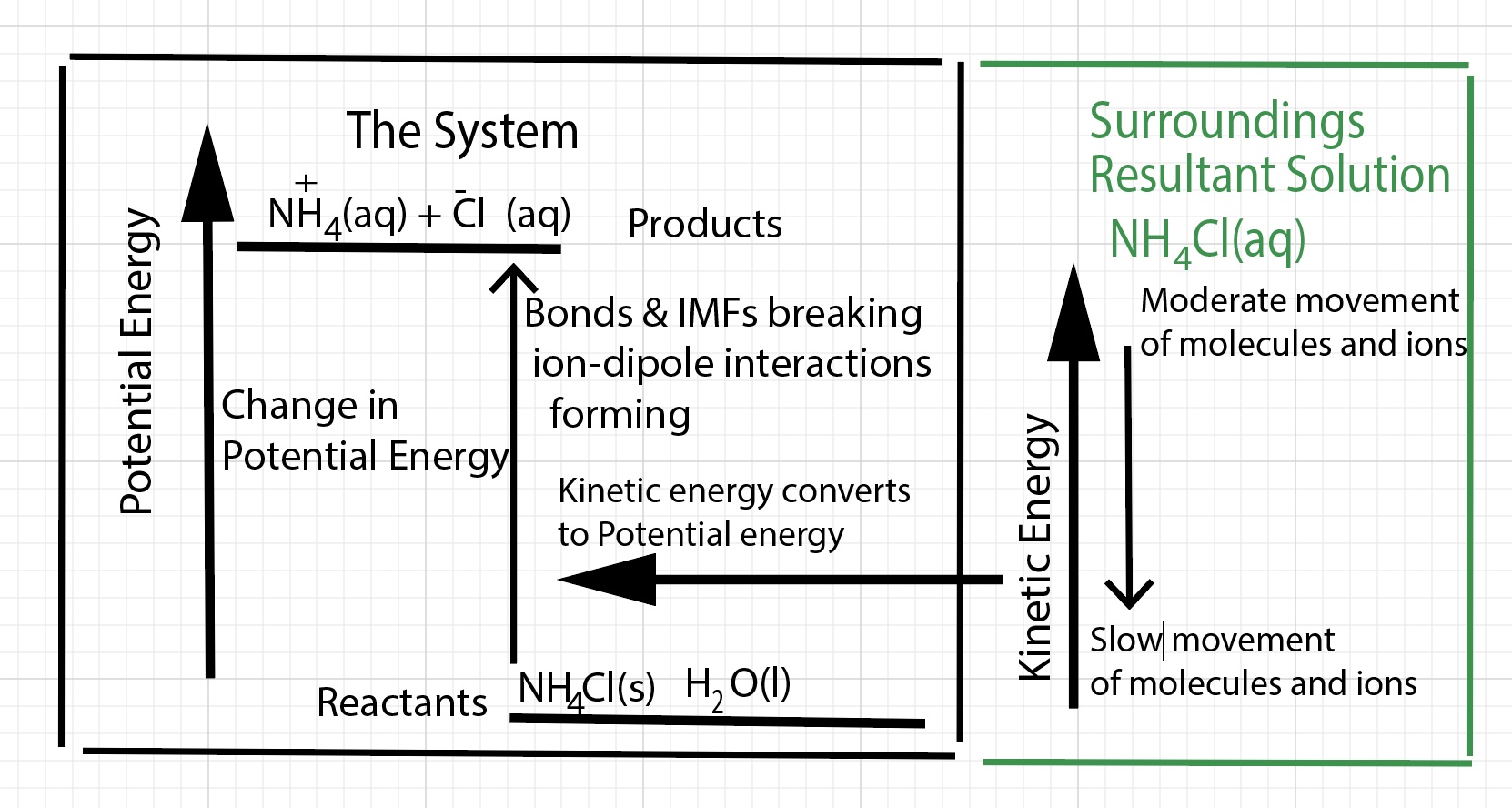
There is a change in enthalpy of the solution. The products are higher in enthalpy compared to the reactants. For this process the change in enthalpy of dissolution is positive, energy is absorbed by the dissolution process, an endothermic process. The change in enthalpy of dissolution is called the heat of solution, ∆H solution or the heat of dissolution.
The calorimeter computer simulation has an animation of a particle view (molecular scene) of the interaction of water molecules with the ions in the solid during the dissolving process. This particle visualization of the dissolving process is a model of energy transfer. As with any model it has limitations and does not capture all of the complexities of the dissolving process.
Energy is taken away from the solution and absorbed by the dissolving process. The energy absorbed goes into separating cations from anions in the solid. Some of the energy goes into separating water molecules. The water molecules have less kinetic energy and slow down. The water molecules hit the thermometer with less impact and the thermometer indicates a lower temperature for the solution.
An animation illustrating energy transfer at the particle level of representation for an endothermic dissolving process. This is a short segment from the simulation. Initially, cations and anions in the solid are vibrating moderately. The cations and anions in the solid are held together by electrostatic forces. An input of energy is required to separate unlike charged particles.When water molecules collide with the cations and anions on the surface of the solid, ion-dipole intermolecular forces form (energy is released). The cations and anions become hydrated (surrounded by water molecules). This releases energy. Most of the energy released by hydration goes into separating cations from anions in the solid. Some of the energy goes into separating water molecules. The dissolution for ammonium chloride requires a significant input of energy - more so than the energy released by hydration. Kinetic energy is transferred from the water molecules to the ions in the solid. The water molecules and ions have less kinetic energy and slow down. The water molecules and ions in solution hit the thermometer with less impact and the thermometer indicates a lower temperature for the solution. When NH4Cl dissolves in water, net energy is absorbed by the dissolving process. <= Currently, Firefox 111.0.1 is doing the best at playing the embedded animations (April, 2023). |
xx

Calculations of ∆H solution for dissolving ammonium chloride (or nitrate) in water using the data from the simulation of mixing NH4Cl(s) in water or mixing NH4NO3(s) in water. The calorimeter simulation has ammonium nitrate.

for this experiment, the solution decreased in temperature, ∆T = (10.26°C - 20.00°C) = -9.74 °C
the solution released energy (the energy of the solution decreased)
qsolution = m c ∆T = 126.0 g x 4.184 J/g°C x (-9.74°C) = -5,135 J
q absorbed by the dissolving process + q released by the solution = 0
q absorbed by the dissolving process = -q released by the solution = -(-5,135 J) = +5,135 J
for a 1:1 stoichiometric ratio
∆H dissolving process = q dissolving process /moles of limiting reagent = +5,135 J / 0.200 moles NH4NO3
= +25,675 J/mol NH4NO3 = +25.7 kJ/mol NH4NO3
∆H solution = ∆H dissolving process
∆H solution = +25.6 kJ/mol NH4NO3
[add data for dissolving ammonium chloride in water here]
Learning Objectives
1. Use experimental data to develop a conceptual understanding of the Law of Conservation of Energy and how to apply it to calorimeter experiments:
q lost + q gain = 0
2. Identify whether a dissolving process is exothermic or endothermic.
3. When an ionic salt dissolves in water, explain how energy is transferred at the particle level.
4. Use enthalpy diagrams to represent dissolving processes. Determine ∆H solution from an enthalpy diagram.
5. Identify what gains thermal energy and what loses thermal energy in a calorimetry experiment.
6. For a physical process, such as dissolving a salt in water, explain how energy is transferred (released or absorbed) at the molecular level.
7. In a calorimetry experiment conducted at constant pressure, given the total mass of the solution, specific heat of the solution, and change in temperature of the solution, calculate the thermal energy gained or released by a solution, q solution = m c ∆T
8. When the calorimetry experiment is carried out under constant pressure conditions, calculate ∆H solution for the dissolving process.
9. Given the change in enthalpy for a reaction, the amounts of reactants, and a balanced chemical equation, calculate the heat exchanged for a reaction.
10. Draw the hydrated ions using six to seven water molecules.
AP Chem Learning Objectives
Learning objective 5.4 The student is able to use conservation of energy to relate the magnitudes of the energy changes occurring in two or more interacting systems, including identification of the systems, the type (heat versus work), or the direction of energy flow.
Learning objective 5.5 The student is able to use conservation of energy to relate the magnitudes of the energy changes when two nonreacting substances are mixed or brought into contact with one another.
Learning objective 5.6 The student is able to use calculations or estimations to relate energy changes associated with heating/cooling a substance to the heat capacity.
Making this presentation interactive - active learning
The instructor should "frame" the instructional activity accompanying this computer simulation. There are several in-class POGIL-like activity to accompany this computer simulation. A recommendation is to use several POGIL activities - calorimetry, lattice energy, coulomb's law, etc. {specify}
A teacher guide and a student activity sheet are available for this lesson.
There are a set of interactive guided-inquiry Power Point slides to accompany this computer simulation. There are clicker questions that can accompany this computer simulation.
Student have difficulties with several concepts associated with thermochemistry (see reference #1 below).
Students do not have difficulty with the idea that the amount of reactants influences the heat exchanged, but they do have difficulty applying this concept.
There are several calorimetry lecture demonstrations that can be used to accompany this computer simulation.
V. References
Abell, Timothy N.; Bretz, Stacey Lowery (2019). Development of the Enthalpy and Entropy in Dissolution and Precipitation Inventory. Journal of Chemical Education, v96 n9 p1804-1812.
Bain, Kinsey; Towns, Marcy H. (2018).Investigation of Undergraduate and Graduate Chemistry Students' Understanding of Thermodynamic Driving Forces in Chemical Reactions and Dissolution. Journal of Chemical Education, v95 (4), p512-520.
Brown, LeMay, Bursten, et al. Chemistry: The Central Science 13th ed. 2015. Pearson: Hoboken, NJ.
Greenbowe, T.J. and Meltzer, D.E. (2003). “Student learning of thermochemical concepts in the context of solution calorimetry.” International Journal of Science Education, 25(7), 779-800.
Johnstone, A. H. (1991). Why is science difficult to learn? things are seldom what they seem. Journal of Computer Assisted Learning, 7(2), 75–83. https://doi.org/10.1111/j.1365-2729.
Johnstone, A. H. (1982). Macro- and micro-chemistry. School Science Review, 64, 377–379.
Malkoc, Ummuhan. (2017). Investigating Teachers' Understanding of the Salt Dissolution Process: A Multi-Media Approach in Education. Turkish Online Journal of Educational Technology - TOJET, v16 n1 p55-71.
Naah, B. M., & Sanger, M. J. (2012). Investigating students’ understanding of the dissolving process. Journal of Science Education and Technology, 22(2), 103–112.
Russell, JW, Kozma, RB, Jones, J Wykoff, N Marx, J Davis (1997). Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts Journal of Chemical Education, 74 (3), 330
*********
©2023 T. Greenbowe, University of Oregon.
