The behavior of gases can be understood knowing the pressure, volume, temperature and number of moles of gas and how these parameters are interrelated. The Kinetic Molecular Model for an ideal gas describes the behavior of gas particles at the atomic/particle level based on a set of assumptions or postulates.
Various physical principles can be investigated using the computer simulation: Boyle's Law, Charles' law, Amonton's Law, Dalton's Law of Partial Pressure, and Graham's Law of Effusion.The computer simulation also provides a representation of what occurs at the molecular level or particle level of gases.
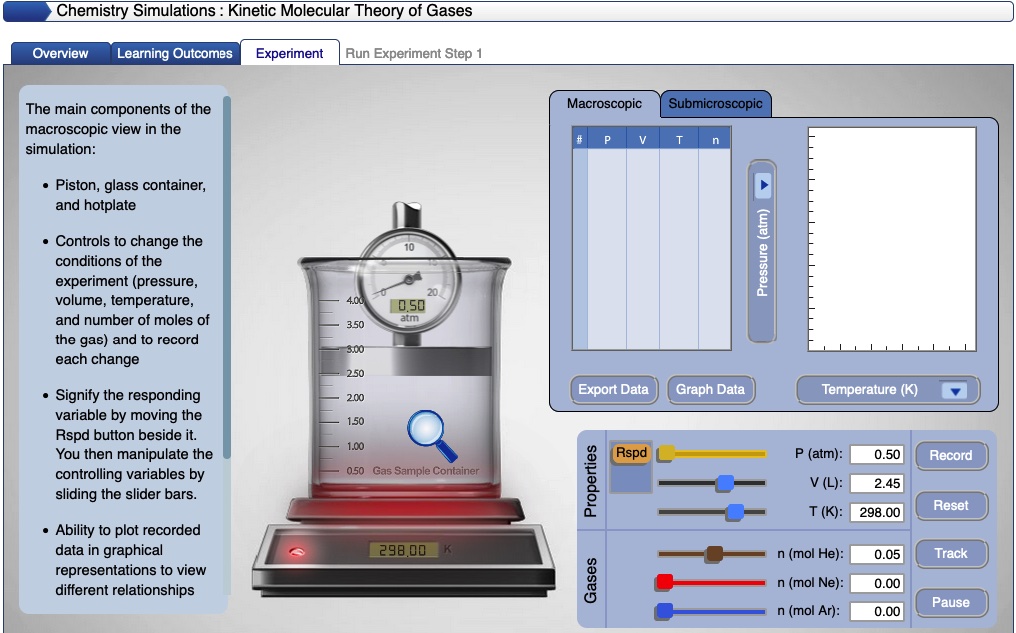
This computer simulation provides an excellent opportunity for students to learn how to ask a research question, design gas law experiments to answer research questions, manipulate variables, collect and analyze data.
©2016 Gelder, Abraham, Greenbowe Kinetic Molecular Theory Computer Simulation Chemistry Interactive Instructional Resources, Oklahoma State University, Oklahoma University, University of Oregon, Pearson: Hoboken, NJ.
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/kmt/KMT.php
An activity sheet and a tutorial is available to accompany this computer simulation.
Web page author: T. Greenbowe, University of Oregon This page is under construction.
Curriculum Notes
This computer simulation provides an illustration of the following postulates of the kinetic molecular theory:
1. Gases consist of particles that are in continuous radom motion.
2. The distance between gas particles is large. The volume occupied by a gas consists mostly of empty space.
3. Gas particles move in straight lines in all directions, colliding frequently with one another and with the walls of the container.
4. No energy is lost by the collision of a gas particle with other gas particles or with the wall of the container.
5. The average kinetic energy for particles is the same for all gases at the the same temperature.
Student Difficulties (Misconceptions) Exhibited with Respect to Gas Laws
1. Gases do not have weight.
2. When a gas expands the particles get larger.
Learning Objectives
1. Design and carry out experiments to answer scientific questions about gases. Determine the relationship in ideal gases between pressure and volume, pressure and temperature, pressure and amount of gas (moles), volume and temperature, and volume and amount of gas (moles).
2. Use the Kinetic Molecular Theory to explain the gas laws. Describer ideal gas behavior in terms of the Kinetic Molecular Theory.
3. Apply the relationship of P, V, T, and n and Dalton's Law to mixtures of gases.
4. Determine the relationship among molar mass, molecular speeds (Graham's law), average kinetic energy, and temperature of gases.
AP Chem Learning Objectives
LO 2.4 The student is able to use the kinetic molecular theory and concepts of intermolecular forces to make predictions about the macroscopic properties of gases, including both ideal and non-ideal behavior.
LO 2.5 The student is able to refine multiple representations of a sample of matter in the gas phase to accurately represent the effect of changes in macroscopic properties on the sample.
LO 2.6 The student can apply mathematical relationships or estimation to determine macroscopic variables for ideal gases.
LO 5.2: The student is able to relate temperature to the motions of particles, either via particulate representations, such as drawings of particles with arrows indicating velocities, and/or via representations of average kinetic energy and distribution of kinetic energies of the particles, such as plots of the Maxwell-Boltzmann distribution.
