All matter has mass. One can measure the mass of an object using a balance. Matter is comprised of atoms, ions, or molecules which we use the general term “particles”. A sample of matter consists of particles that can be vibrating, rotating and moving through the space. At the particle level, a sample of matter possesses kinetic energy. We can represent the arrangement of particles in solids, liquids, and gases using particle diagrams and animations at the particle level.
A short presentation representing particles in solids, liquids and gases using animations
https://www.youtube.com/watch?v=bwGim-eceS8&t=21s
This page is under construction. Web page Author: Tom Greenbowe, University of Oregon.
Energy - the capacity to do work or the ability to transfer "heat energy".
Thermal energy - the microscopic energy of atoms and molecules within an object. The thermal energy of an object is due to the motion and or vibrations and or rotations of its atoms and or molecules.Thermal energy is a state variable. A solid object at a specific temperature will have thermal energy due to the fact that atoms or molecules of the object are vibrating.
Thermal energy transfer - the symbol "Q" and or "q" represents the quantity of thermal energy transferred between two objects at different temperatures, during a phase change of a pure substance or during a chemical reaction between the "system" and "surrounding".
Temperature is a state variable that quantifies the "hotness" or "coldness" of an object relative to a standard. A measurement of temperature is associated with (or a function of) the average vibration or speed of molecules, which is related to the average kinetic energy of the atoms or molecules in a substance - the average KE = (1/2)mv2. Temperature is not a direct measurement of energy. Knowing the temperature of a sample of gas, one can calculate the translational kinetic energy of the sample of gas. Note, atoms and or molecules of a solid are not moving from point A to point B. The atoms and or molecules are vibrating - which is not translational energy.
If we compare the temperature of a hot cup of water to a cold cup of water using a thermometer, we observe that the hot cup of water has a higher temperature. At the particle level, there are water molecules vibrating, rotating and moving through the liquid. The water molecules in the hot water are striking the surface of the thermometer more vigorously compared to the water molecules in the cold water.
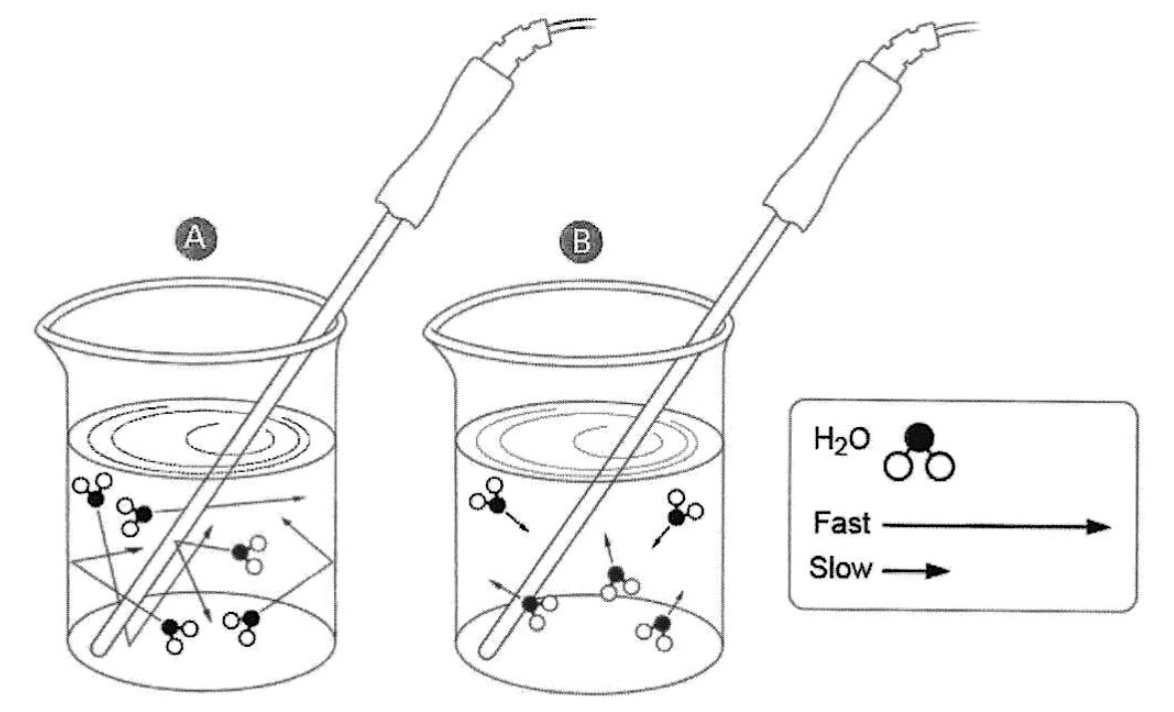
Two beakers, A and B, with the same volume of water at different temperatures. Particle level diagram of a representation of the relative speeds of water molecules in hot water, diagram A molecules moving faster, compared to cold water, diagram B molecules moving slower. Each sample of water has a thermometer probe to measure the temperature of the water. Note that the size of the water molecules are not to scale. Image is from Advanced Chemistry Inquiry Through Inquiry POGIL, Pasco Scientific, out of print.
The water's temperature, as reflected by the thermometer's reading, is a measure of the "hotness" or "coldness" of the water near the thermometer, relative to a standard. A thermometer placed in a cup of water provides evidence supporting the fact that the water molecules are moving at a higher average speed in the hot water compared to the cold water. The average amount of kinetic energy possessed by the water molecules in a local volume around the thermometer can be qualitatively inferred from the measurement of temperature.
In the cup of hot water, some water molecules are moving faster than others because there is distribution of speeds of the water molecules. In a cup of cold water some water molecules are moving faster than others because there is distribution of speeds of the water molecules. Comparing the distribution of speeds of water molecules at two different temperatures where T2 is a higher temperature compared to T1, there are more molecules moving faster in the higher temperature sample compared to the lower temperature sample.
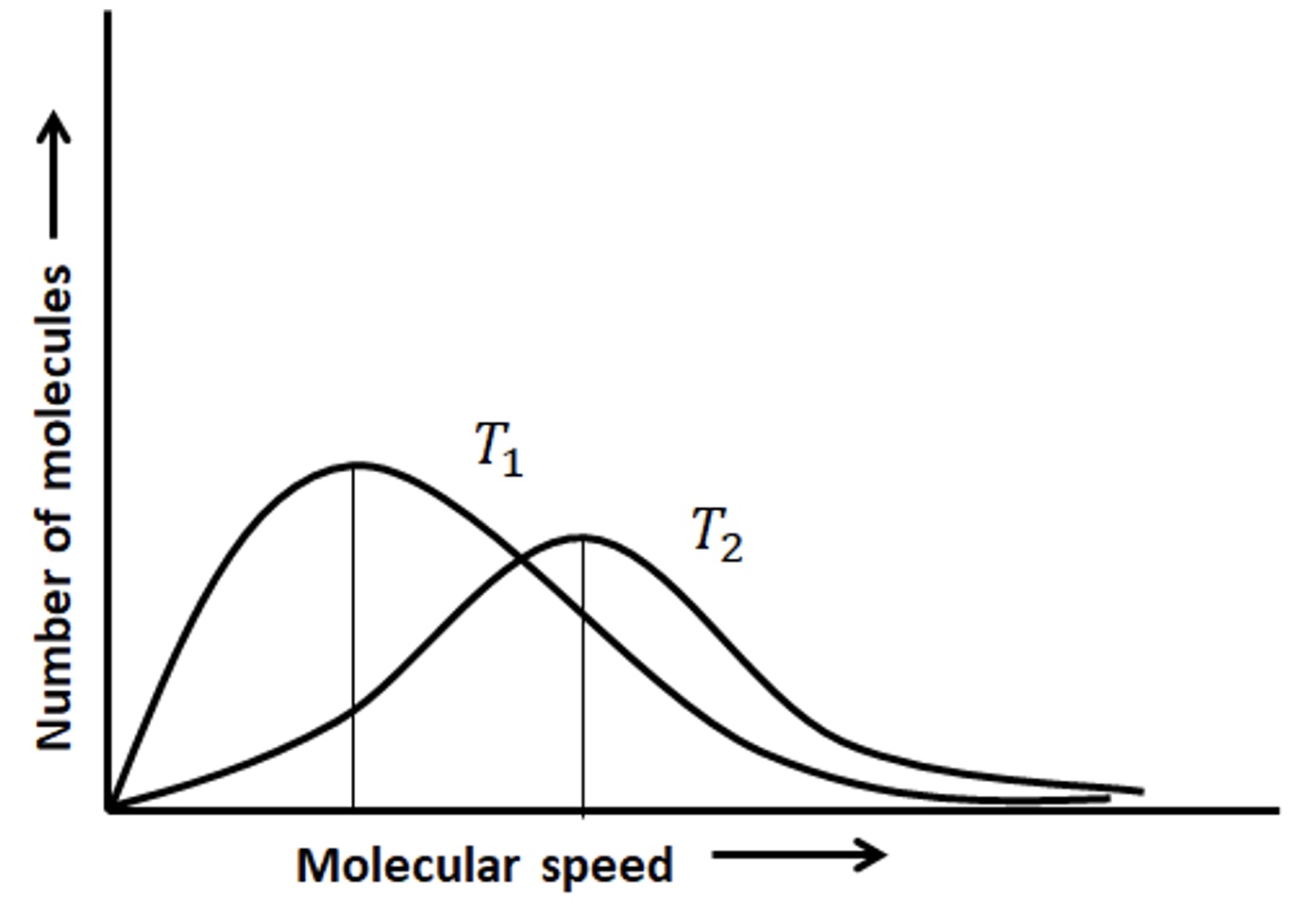
When there is distribution of molecular speeds there is a distribution of kinetic energies. There is a most probable energy at the apex of each curve. Comparing the same type of gas molecules (same molar mass) at two different temperatures, the sample with the higher temperature has a greater higher most probable kinetic energy compared to the sample at a lower temperature.
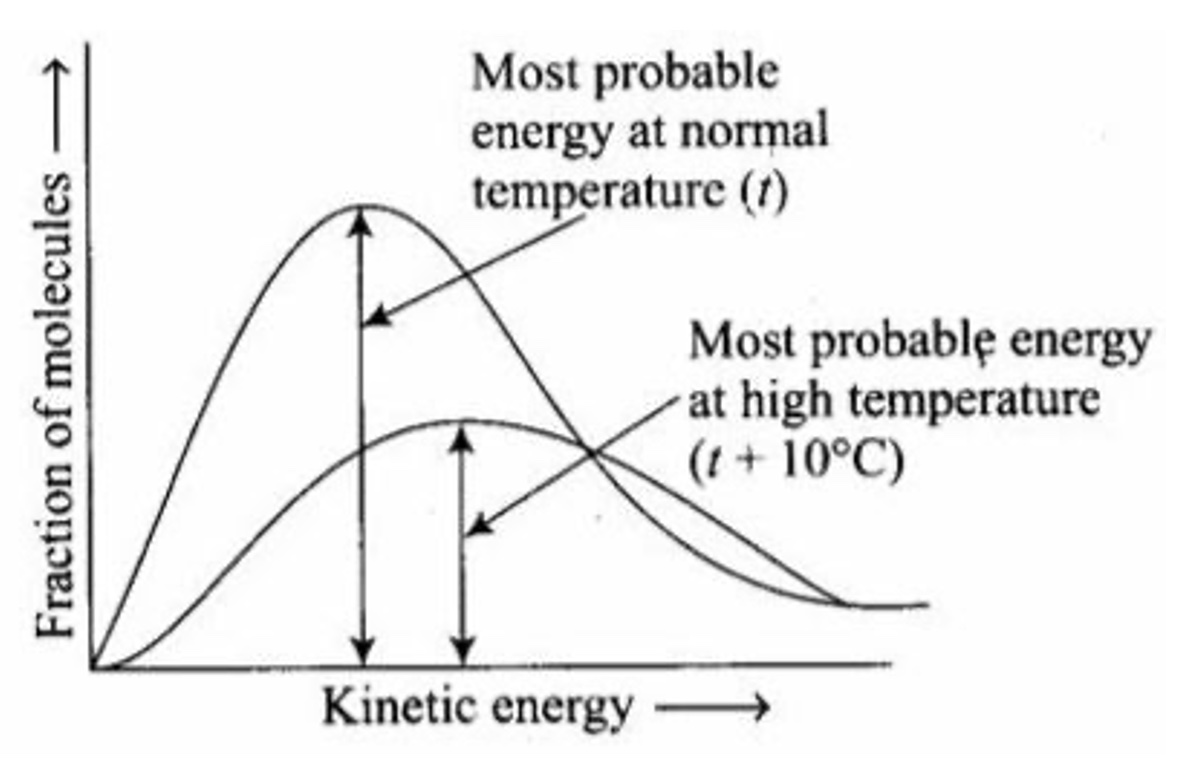
Comparing the same type of molecules, in the case water molecules (same molar mass), at two different temperatures, the sample with the higher temperature has a greater distribution of kinetic energies compared to the sample of water at a lower temperature. There is a most probable energy at the apex of each curve. The apex of the distribution curve of the cold water is at a lower most probable kinetic energy compared to the hot water curve's most probable energy. A sample of hot water has a higher most probable average kinetic energy compared to the most probable average kinetic energy of the cold water.
The units of temperature are degrees Celsius, Kelvin, or degrees Fahrenheit. Note, temperature is not a direct measurement of the average energy for solids or liquids! One of the units of energy is Joules. joules and Kelvins are different units.Temperature is related (or is a function of) to the thermal energy per molecule.
Returning to the speed of gas molecules and the distribution of gas molecules
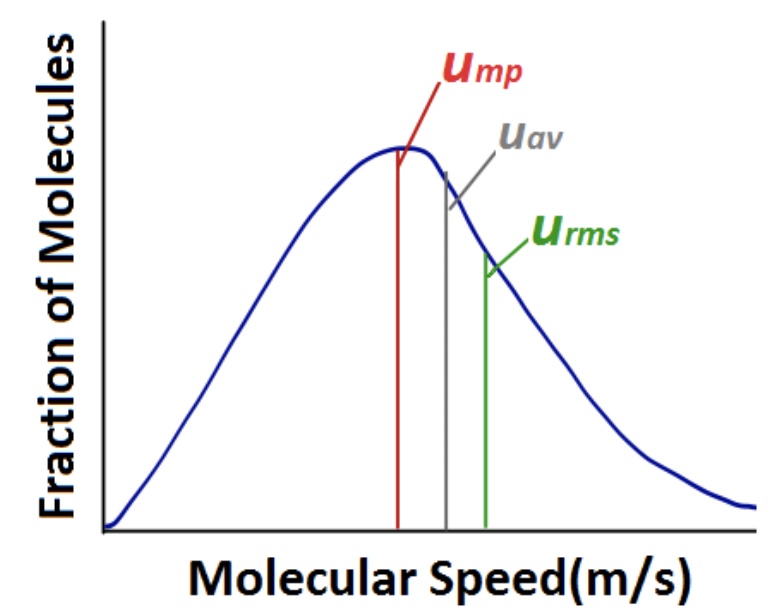
When analyzing a graph of the distribution of molecular speeds of a gas, one must bre aware of several termsused to describe molecular speed. The most probable speed (ump) is the speed of the largest number of molecules, and corresponds to the peak (apex) of a distribution. The average speed (uav) is the mean speed of all gas molecules in the sample. The root mean square speed (urms) corresponds to the speed of gas molecules having exactly the same kinetic energy as the average kinetic energy of the sample.
A computer simulation of a sample of gas partilces confined to a two-dimensional area and a graph of the speed distribution of the particles
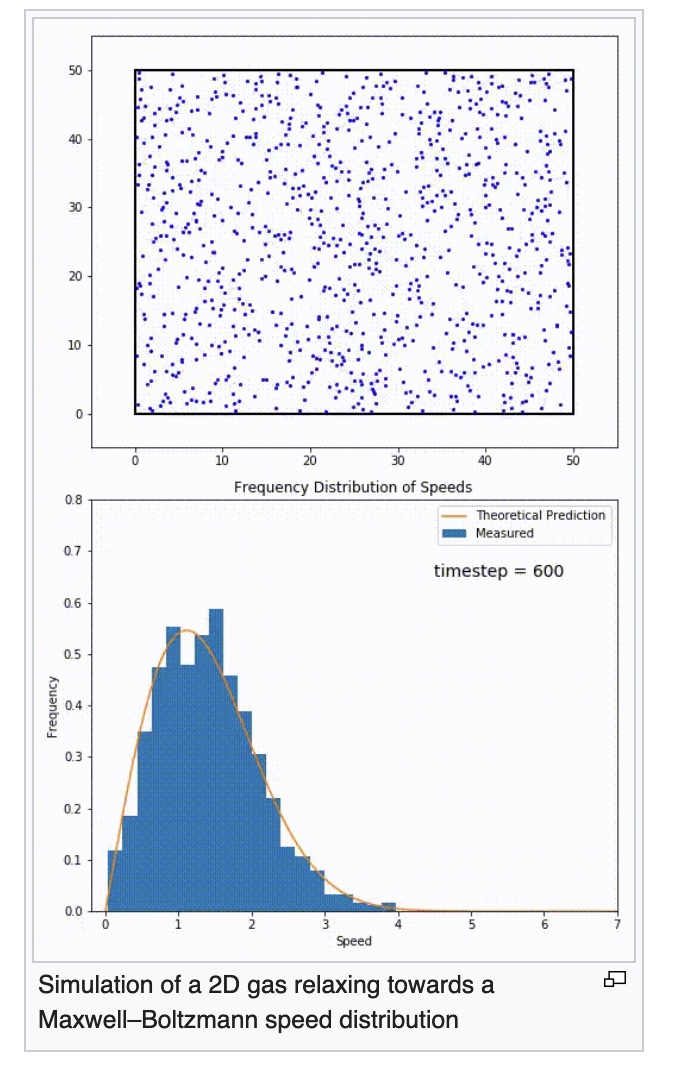
https://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution
We can define another unit of energy, translational energy. For a single molecule of mass, m, and velocity, v
E = (1/2)mv2
For a gas, average translational kinetic energy Etl av per molecule = (3/2)kBT where kB is the Boltzman constant and T is temperature in units of Kelvin.
Since average kinetic energy is related both to the absolute temperature and the molecular speed, we can combine the two equations above to determine the rms speed.
For a gas, a temperature measurement (by a thermometer) does afford a route to calculate the average translational kinetic energy. A thermometer does NOT directly measure average translational kinetic energy.
The rms speed of gas molecules is proportional to the temperature. We can further develop this equation by multiplying the numerator and denominator by Avogadro’s constant (NA) to yield an equation incorporating the gas constant (R) and molar mass (M) of a gas.
This equation demonstrates that the rms speed of gas molecules is related to the molar mass of the gas and the temperature (units of Kelvin) of the gas.
Comparing several gases of different molar mass at the same temperature, even though the gases have the same average kinetic energy, the gas with the smaller molar mass will have a higher rms speed compared to a gas with a larger molar mass.
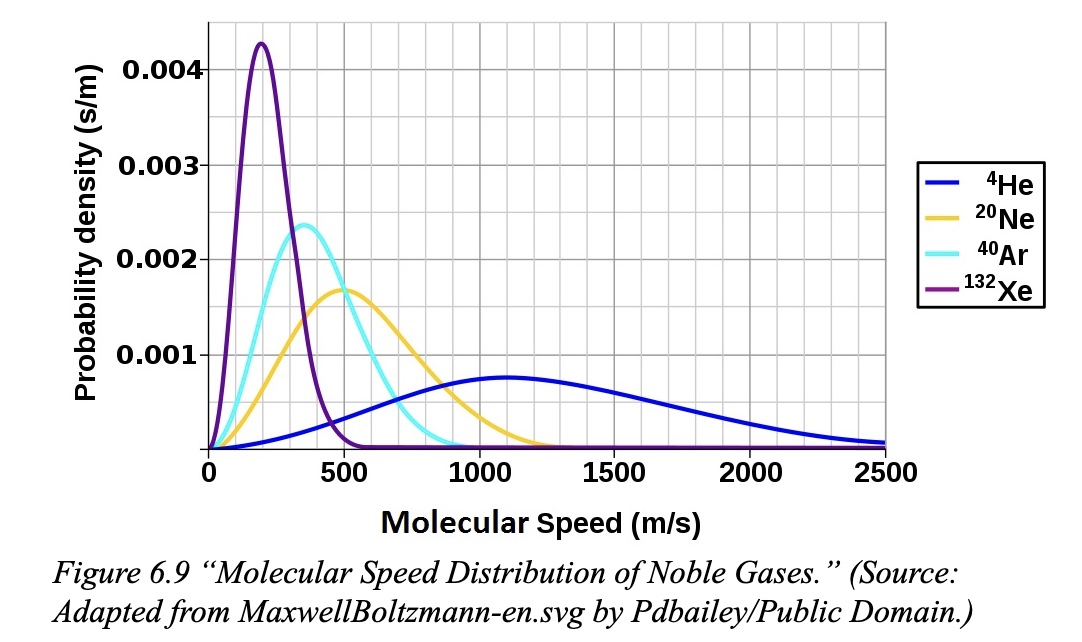
A graph of number of particles versus kinetic energy of helium gas at three different temperatures shows different Maxwell-Boltzman distributions. Next, comparing several gases of different molar mass at the same temperature shows gases at the same temperature have the same average kinetic energy and have the same Maxwell-Boltzman distribution. Note that the shape of distributions of particles versus speed graph is different from the shape of distribution of particles versus kinetic energy graph.
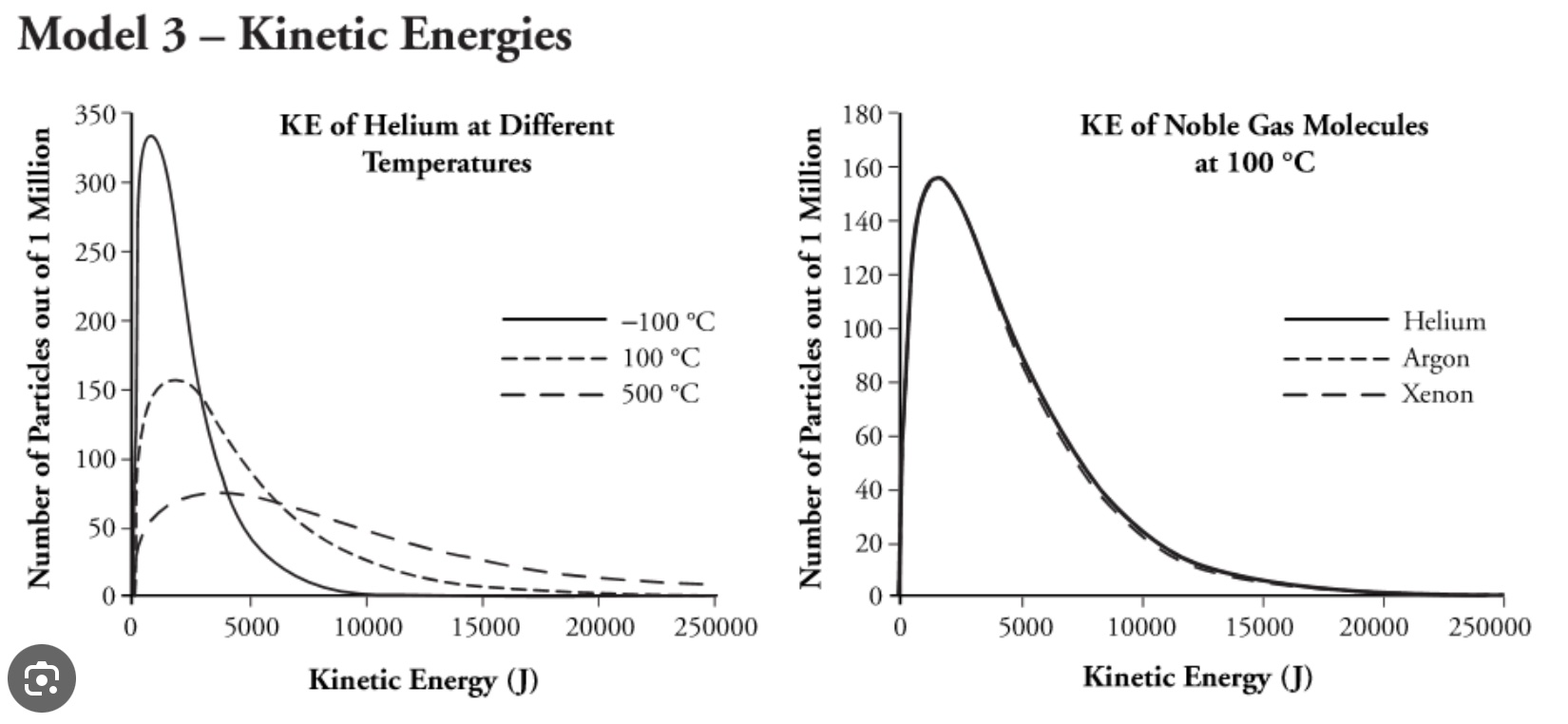
Graphs are from the POGIL AP Chemistry, L. Trout editor, Flinn Scientific.
Calorimetry Experiments
In chemistry calorimeter experiments, we are working with solids, liquids, and aqueous solutions. The energy exchanged in calorimetry experiments is thermal energy.
The energy transferred between a system and surrounding as a consequence of a temperature difference or as a consequence of a change in physical state (melting, boiling, etc.) of a pure substance can be represented as "q". The process of energy transferred between system and surrounding through atom and or molecular level collisions is called thermal interaction.
For a calorimetry experiment
when q > 0 we assume thermal energy is added
when q< 0 we assume thermal energy has been released or extracted
Heat. A better term to use is Heating. The word "heat" is not well understood and is misused by science teachers. Heating is a process, a mechanism for transferring energy from a hot object to a cooler object or during a phase change. Heat is NOT a form or type of energy. Heat is NOT a state function or a state variable. Heat is NOT thermal energy. Heat is not a form of storing energy. Heat is NOT an object. An object at a specific temperature cannot possess heat. If an object is at a certain temperature, one cannot say anything about the heat of that object. If an object changes temperature, one cannot calculate a change in heat, ∆q. For example, when someone says, "The stove is heating the pot. Do not touch the pot because it is hot." With respect to science, heating does not mean the temperatuare change of the pot.
The symbol, "q" can be identified as "heating" - a physical quantity that can be calculated, not measured.
For an object undergoing a change in temperature, q = mass x specific heat x change in temperature
It is important NOT to associate an observed temperature increase of an object with heat (Knight, 2022).
Particle diagrams represent a view of matter in a small area or volume.
https://www.youtube.com/watch?v=_5AZwrTkQNA
xxxx
Conduction of Energy in a Metal Using a Particle View - Iron atoms
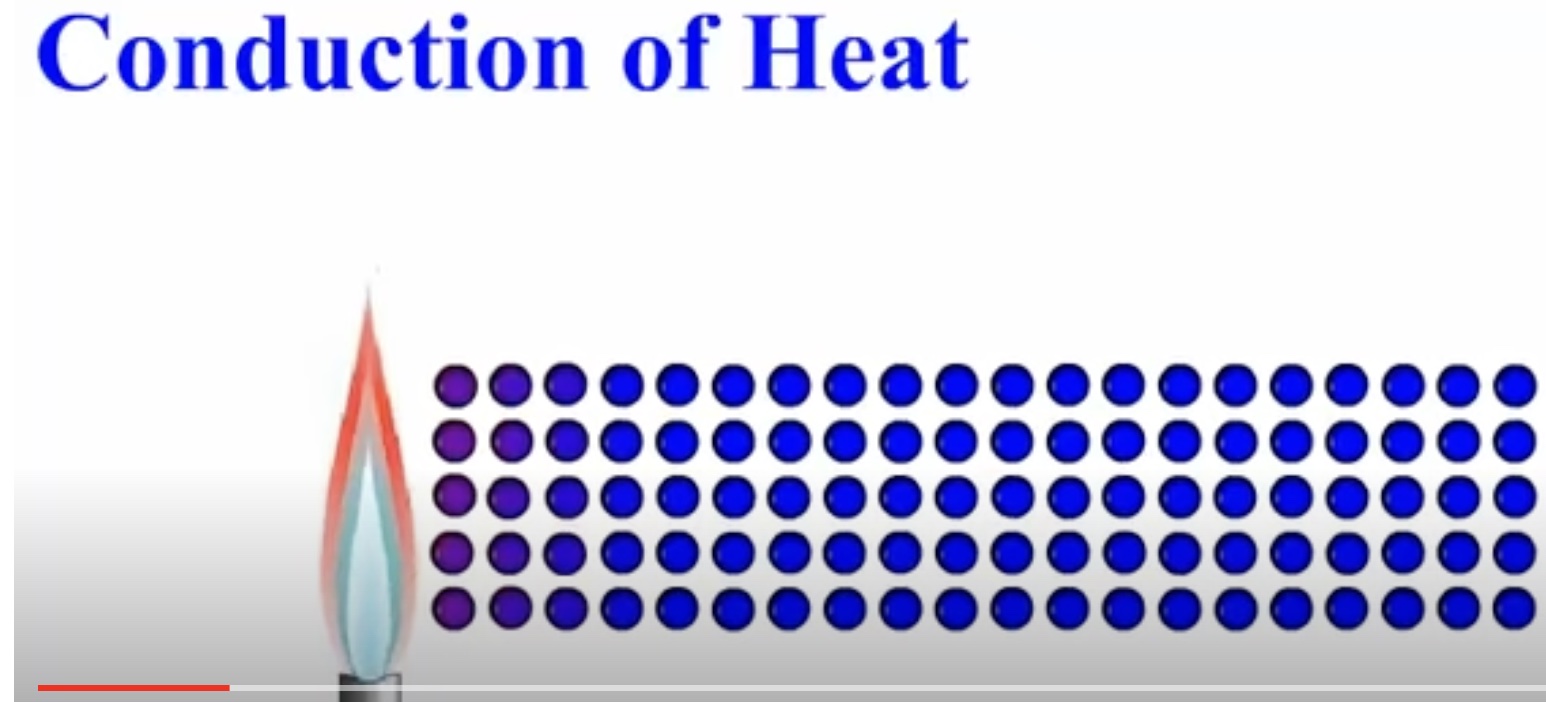
Atom vibrations in a metal
Vibrations of atoms in a block of metal are proportional to the temperature of the metal.
In a block of metal, as the vibration of atoms increases, the temperature of the block of metal increases.
Temperature is related to the average kinetic energy of a group of atoms in a local region in a block of metal. In the case of a piece of metal, the metal atoms are vibrating moderately at room temperature and vigorously at a higher temperature.
A computer animation of atoms in a block of metal.
ACS AACT. Atom vibrations in a. metal
https://www.acs.org/education/resources/k-8/inquiryinaction/fifth-grade/particles-solid-hammer.html
Conduction of Heat in a Metal. Animation of particles (atoms) in a metal when heated.
https://www.youtube.com/watch?v=qW59Y9lJso8
This animation depicts a very small area of the metal. Circles represent atoms in a metal. At room temperature the atoms are vibrating slowly. The atoms are given a blue color. A flame is brought near an end of the metal. The atoms at the end of the metal begin to vibrate faster. The color of the atoms, at the end of the metal near the flame, change color from blue to red signifying a higher temperature. The atoms vibrating faster collide with neighboring atoms – cooler in temperature and vibrating less vigorously. After the collision, the faster vibrating atoms loose some vibrations and the neighboring atoms begin to vibrate faster. Transfer of energy in a metal occurs through the collisions of atoms having different degrees of vibrations (faster versus slower) which is correlated with a difference of temperature.
There are three common forms of kinetic energy - vibrational kinetic energy, rotational kinetic energy and translational kinetic energy. Translational kinetic energy is kinetic energy of an object (or particle) from one location to another - movement. A ball moving through space has translational kinetic energy. An object oscillating or vibrating about a fixed position has vibrational kinetic energy.
The problem with using the word "Heat" in a scientific explanation
Heat is not an object. Heat is not a property of an object. Thermal energy exchange is a process. It is important NOT to associate an observed temperature increase with heat (Knight, 2022).
If there is a difference in difference in temperature of an object, on can infer thermal energy was exchanged. The object either absorbed thermal energy or released thermal energy.
References
