Select various metals to test in aqueous M2+ solutions. Build an activity series of metals based upon observations of whether or not a metal reacts with a M2+aqueous solution. Option to view a computer animation at the particle level of the interaction of the M2+ion with the metal electrode. Based on observations, write the the oxidation-reduction half-reactions.
Metals: magnesium, zinc, copper, silver
Aqueous solutions: magnesium nitrate, zinc nitrate, copper(II) nitrate, silver nitrate
Zinc metal is placed in samples 0.5 M aqueous solutions of magnesium nitrate, zinc nitrate, copper(II) nitrate, and silver nitrate.

After a few minutes the zinc metal is lifted out of the solutions and a copper metal is plated on zinc that was placed in the copper(II) nitrate soluiton and silver is plated on the zinc that was in the silver nitrate solution.
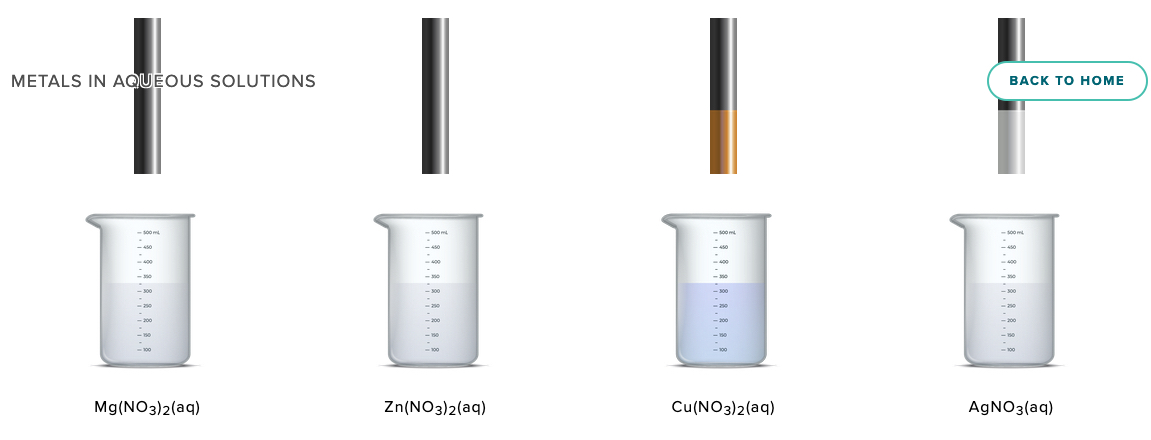
Activity Series of Metals Computer Simulation Metals in Aqueous Solution ACS AACT New Simulation
©2021 Thomas Greenbowe University of Oregon, John Gelder Oklahoma State University, Adam Boyd AACT, Monica Wixon AACT. American Association of Chemistry Teachers, American Chemical Society: Washington, DC.
Web page Author: T. Greenbowe, University of Oregon. This page is under construction.
Animation of the oxidation-reduction half-reactions at the surface of the zinc metal in copper(II) nitrate solution. Particle level animation.
Zn --> Zn2+(aq) + 2e-
Cu2+(aq) + 2e- --> Cu(s)
In this activity, students will run simulated tests of various metals in aqueous solutions to determine the relative reactivity of these metals. A total of eight metals will be observed in various combinations with the corresponding metal nitrate solutions and hydrochloric acid. Students will interpret the data collected to construct an activity series of the elements used in this simulation (AACT, 2021).
Teaching Tip Do this experiment just before going into electrochemical cells. Students need to be able to identify the more active metal in simple electrochemical cells. Order the metals from least active to most active
Silver least acive
Copper
Zinc
Magnesium most active
This order matches the order of metals on a table of standard reduction potentials.
Rather than lecturing students on the activity series of metals why not let the students make observations and develop their own activity series of metals? Students can compare their activity series with the one in their textbook. A student activity sheet developed by Gelder, Abraham and Greenbowe is available to use with this computer simulation and is available to down load. See side menu.
Curriculum Notes
When the student activity sheet /tutorial is used with computer simulation and the computer animations representing reactions at the particle level (atom level), and when students have the opportunity to do an activity series of metal experiment in the laboratory it is an effective way of exposing students to all three levels of representation in Alex Johnstone's triangle: microscopic, symbolic and macroscopic levels of representation. Note, Alex Johnstone stressed instructors should not have students view all three levels simultaneously, but rather "slide" on one side of the triangle at a time..
Learning Objectives
1. Predict and/or justify trends in atomic properties of metals based on location on the periodic table and/or the shell model.
2. Make observations involving metals in metal ion solutions with respect to whether a reaction occurred or not.
3. Construct an activity series of metals. Justify with evidence the order of chemical reactivity of metals.
4. Identify oxidation-reduction reactions and justify the identification in terms of electron transfer.
5. Make qualitative or quantitative predictions about galvanic or electrolytic reactions based on half-cell reactions and standard reduction potentials.
References
Abraham, M.; Gelder, J.; Greenbowe, T. (2007). During Class Inventions and Computer Lab Activities for First and Second Semester General Chemistry. Hayden-McNeil: Plymouth, MI.
Kieffer, W.F. (1950). The activity series of metals. J. Chem. Educ., 27 (12), p 659. DOI: 10.1021/ed027p659.
Shakhashiri, Bassam Z. “An Activity Series: Zinc, Copper, and Silver Half Cells,” Chemical Demonstrations: A Handbook for Teachers of Chemistry, Volume 4 (Madison: The University of Wisconsin Press, 1992), pp. 101−106.
