This simple demonstration illustrates the conductivity properties of a strong electrolyte, a weak electrolyte, and a non-electrolyte. Deionized water fails to light the light bulb in the conductivity tester. A light bulb conductivity tester does not light up at all when the electrodes are inserted into either solid granular sodium chloride or solid granular sucrose. However, when solid NaCl dissolves in water, the sodium chloride solution lights the bulb brightly. When solid sucrose dissolves in water the sucrose solution fails to light the bulb. A 1M acetic acid solution makes the bulb glow dimly.
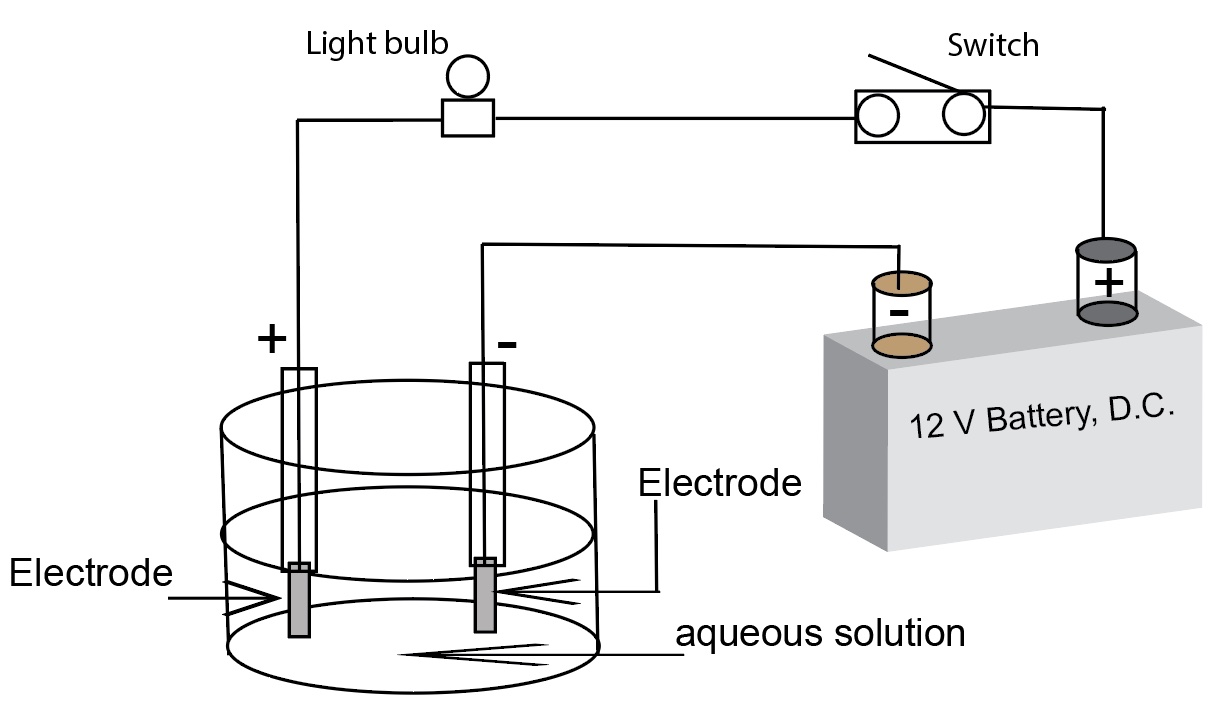
A diagram of a simple conductivity apparatus
Curriculum Notes
This demonstration can be used to illustrate how the number of ion particles in solution classify an aqueous solution as a strong electrolyte , weak electrolyte or non-electrolyte. This demonstration can be used to help discuss topics such as the solution process, intermolecular forces, or the relative strengths of chemical bonds vs. IMF. The effectiveness of this demonstration is increased when 1) students make their own predictions, observations, and inferences; 2) draw particle level diagrams of all of the tested solutions and solids; and 3) answer questions and write explanations about solutions.
Discussion
Substances only conduct electricity when they contain mobile charged particles. The loosely held outer shell electrons of metals are sufficiently mobile to conduct electricity, so the screwdriver tests positive for conductivity. Sodium chloride contains both sodium and chloride ions, but in the solid state they are locked in place in the crystal lattice and are therefore unavailable to conduct electricity. But when sodium chloride is dissolved in water, the crystal lattice is disrupted, and the solvated cations and anions are free to move in the solution. Using a simple representation, the electrodes are connected to a direct current battery. One electrode is connected to the positive terminal of the battery. The other electrode is connected to the negative terminal of the battery. The positively charged cations in solution are attracted to the negatively charged electrode. The negatively charged anions in solution are attracted to the positively charged electrode. Cations and anions migrating in opposite directions constitutes a current. The circuit in the conductivity apparatus will conduct electricity when ions are free to move in an aqueous solution. Sodium chloride dissolves in water to form an aqueous solution, NaCl(aq), which strongly conducts electricity. The NaCl is solution is called a strong electrolyte. Solutions having lots of ions will light the light bulb brightly.
All of the bonds in the sucrose molecule are strong covalent bonds. Therefore, there are no charged particles present to conduct electricity either in the solid state or in solution. Substances like sucrose which do not conduct electricity in aqueous solution are called non-electrolytes.
Acetic acid is a carboxylic acid having an acidic hydrogen bonded to an oxygen atom. There are other hydrogen atoms in acetic acid, but these are bonded to carbon atoms. The hydrogen atom of acetic acid that is bonded to an oxygen atom is loosely bonded. Therefore, when placed in water, polar water molecules occasionally detach a partial positively charged hydrogen ion from the rest of the molecule creating a pair of ions, hydronium ion and the acetate ion. The acetate ion is resonance stabilized.
[chemical equation here]
Since only a small percentage of the acetic acid molecules exist in the dissociated state at any given time, acetic acid solutions only conduct electricity weakly. Substances like acetic acid which weakly conduct electricity in aqueous solution are called weak electrolytes. Solutions having only a few ions will light the light bulb dimly.
If you would like to show your students that pure ionic substances in the liquid state (melts) conduct electricity, check out the "Conductivity Of Ammonium Acetate" demo.
Authors: Randy Sullivan and Tom Greenbowe, University of Oregon. This page is under construction.
We strongly suggest instructors include a short animation, the particle level of representation, of cations and anions, in solution, migrating in opposite directions in ordder to drive home the point that movement of cations and anions constitutes an electrical current.
Safety Precautions
None of the chemicals used in this demo are hazardous. The main hazard is the conductivity apparatus. The electrodes are connected straight to 120 volts of AC current. Severe shock can result if you touch them when the circuit is "live." Be sure to turn the power strip off when there is any chance that you might touch the electrodes.
Conductivity apparatus safety comments and cautions when using the simple apparatus connected to 120 Volts AC outlet
The person doing the demonstration (instructor or demonstrator) needs to wear a pair of rubber gloves before plugging the conductivity apparatus electrical cord into an AC electrical outlet. The gloves are kept on through out the entire experiment and while the apparatus is plugged in. The instructor or demonstrator shall wear safety googles.
To prevent electric shock, the electrodes are not to be touched while the apparatus is plugged into a 120 volts AC outlet. The apparatus is not to be left on unattended. In any demonstration involving “live” electrical contacts, the apparatus must be disconnected from the power source except when actually doing the demonstration and making observations.
Conductivity Apparatus Notes
A good conductivity apparatus is necessary for this demonstration, one that indicates more than not lit, dimly lit, and brightly lit bulb. Students grasp several concepts when the conductivity apparatus is illustrated as having a direct current battery with a positve pole and a negative pole. Students readily argee the positive cations in solution will be attracted to the negative electrode and the negative anions will be attracted to the positive electrode. However, there are problems with polarization about the electrodes within a few seconds of operation. Most simple conductivity testers , using bare wires, are plugged directly into a 120 volt AC outlet. This is a safety hazzard. the Journal of Chemical Education has published several articles about homemade conductivity testers, complete with materials and circuit diagrams. All of theses provide some degree of qualitative indications (bar graphs, multiple lights) that goes beyond bright and dim and not lit categories.
Materials
- 1 150 mL beaker containing about 20g of NaCl
- 1 150 mL beaker containing about 20g of sucrose
- 500 mL of deionized water
- 200 mL of 1M acetic acid
- 3 400 mL beakers
- 2 stirring rods
- conductivity tester mounted on ring stand
- power strip
- wash bottle containing deionized water for rinsing electrodes
- large crystallization dish to catch rinse water
- screwdriver with insulated handle
- paper towels
Procedure
- Plug in power strip to electric outlet and plug in conductivity tester to power strip. Make sure that the power strip is turned off!
- Turn on the power strip. Short the electrodes with the blade of the screwdriver to show the students what a positive conductivity result looks like. The bulb lights up. Turn off the power strip.
- Turn on the power strip and insert the electrodes into the NaCl crystals. The bulb does not light. Turn off the power strip. Rinse and dry the electrodes.
- Turn on the power strip and insert the electrodes into the sucrose crystals. The bulb does not light. Turn off the power strip. Rinse the electrodes.
- Pour about 200 mL of deionized water into one of the 400 mL beakers. Turn on the power strip and insert the electrodes into the water. The bulb does not light. Turn off the power strip.
- Add the NaCl to the water in the beaker and stir. Turn on the power strip and insert the electrodes into the NaCl solution. The bulb lights up. Turn off the power strip. Rinse the electrodes.
- Pour about 200 mL of deionized water into one the other 400 mL beakers and add the sucrose. Stir. Turn on the power strip and insert the electrodes into the sucrose solution. The bulb does not light. Turn off the power strip. Rinse the electrodes.
- Pour about 200 mL of 1M acetic acid into the remaining 400 mL beaker. Turn on the power strip and insert the electrodes into the acetic acid solution. The bulb glows dimly. Turn off the power strip.
Estimated time to do the demonstration
Allow about 15 minutes to perform this demonstration.
One day of lead time is required for this project.
Learning Objectives
1. Explain why water is a polar molecule and how five or six water molecules interact with substances to participate in the dissolving process.
2. From physics, apply the concepts of "unlike charges attract", dipole moment, and vectors to help explain why polar substances and soluble ionic substances dissolve in water.
3.Categorize an aqueous solution as being a strong electrolyte, weak electrolyte, or non-electrolyte.
4. Represent solid ionic substances, polar covalent substances, and covalent substances using particle level diagrams.
5. Represent the dissolving process of solid ionic substances and polar covalent substances, and covalent substances using particle level diagrams and chemical equations
6. Clearly illustrate and explain the distinctions among strong electrolyte, weak electrolyte, and non-electrolyte solutions.
7. Identify weak acids, strong acids, weak bases, and strong bases.
