Solutions
Dissolving an Ionic Salt in Water and Energetics
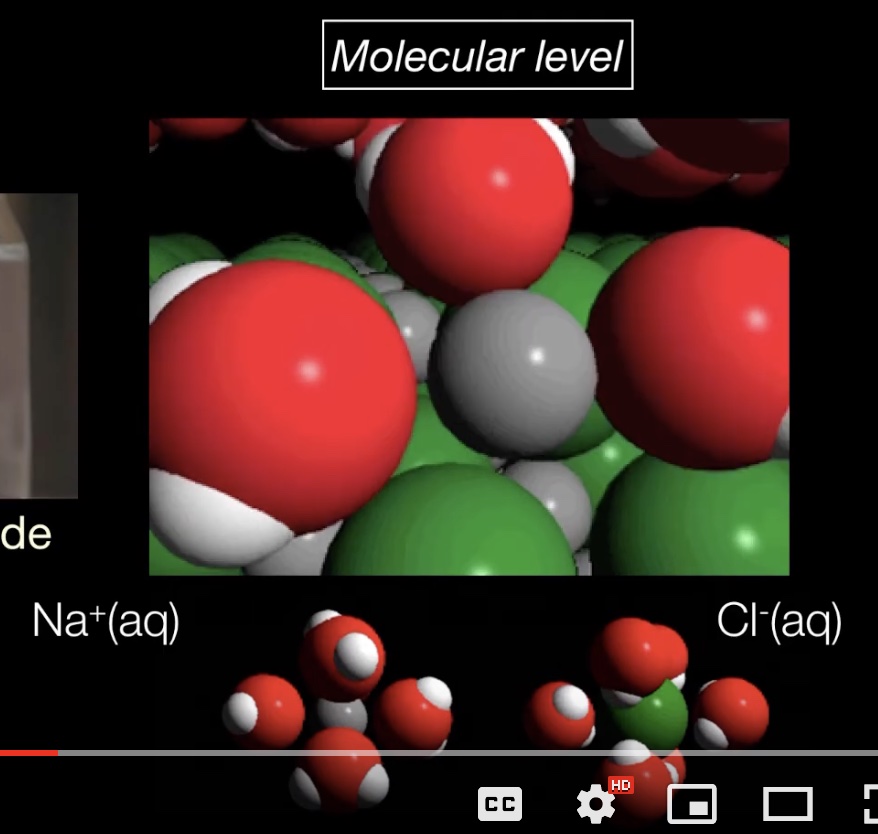
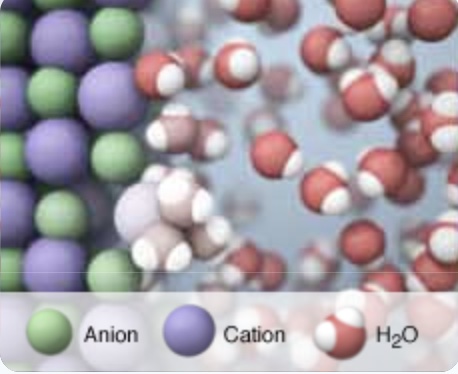
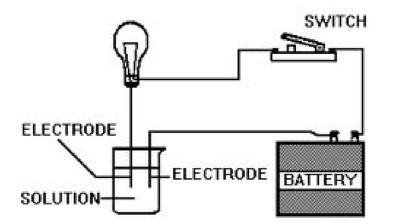
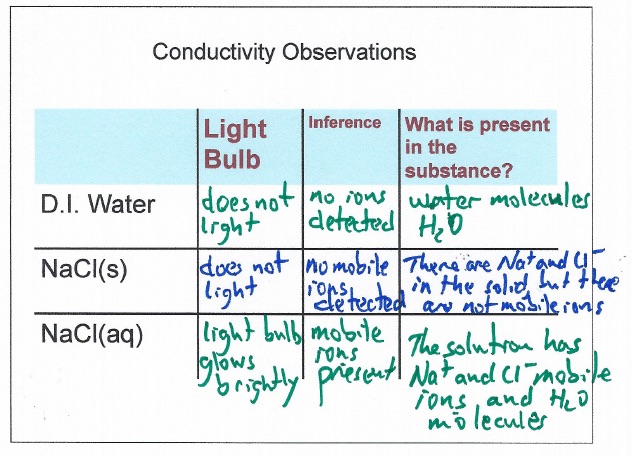
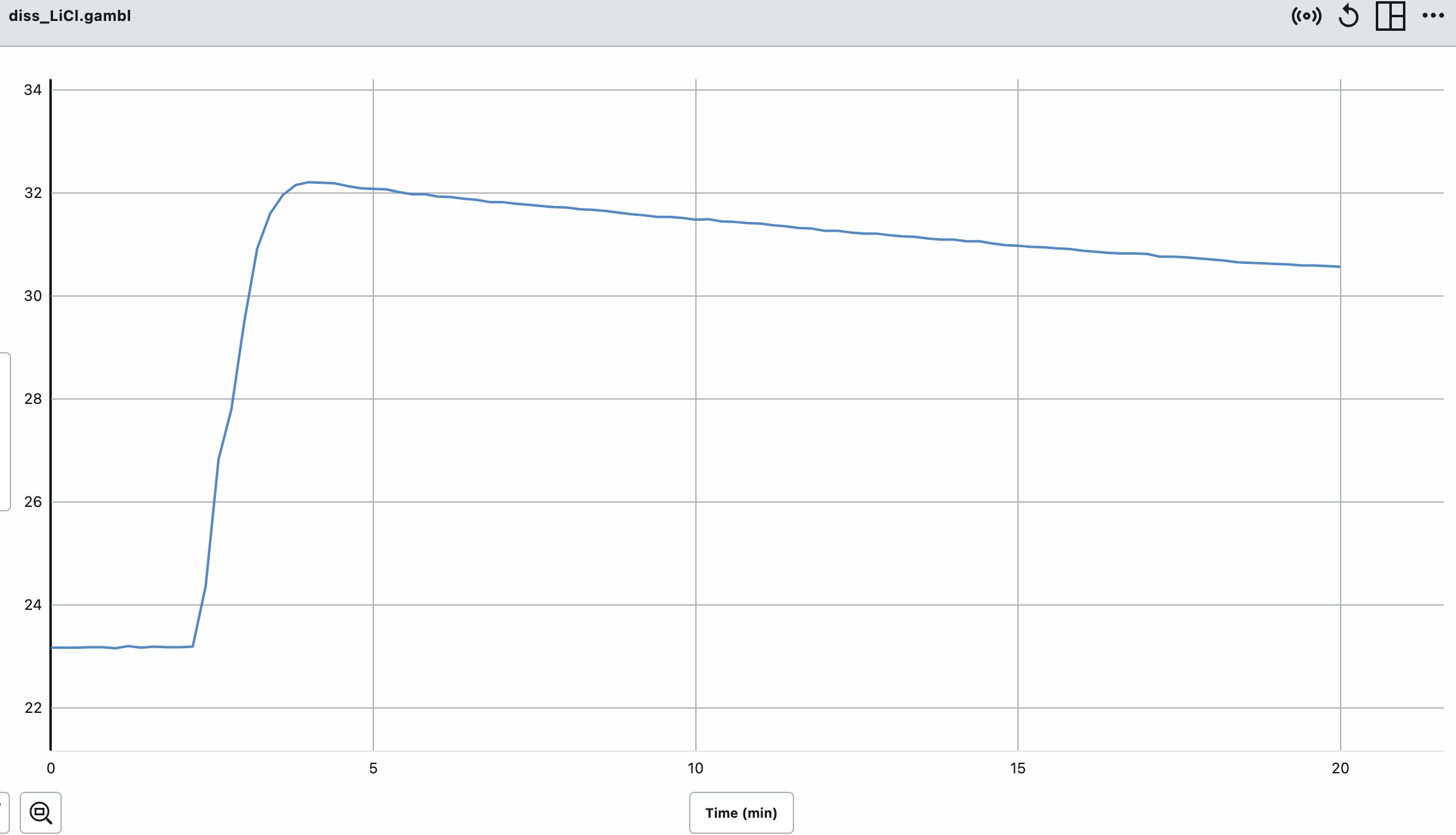
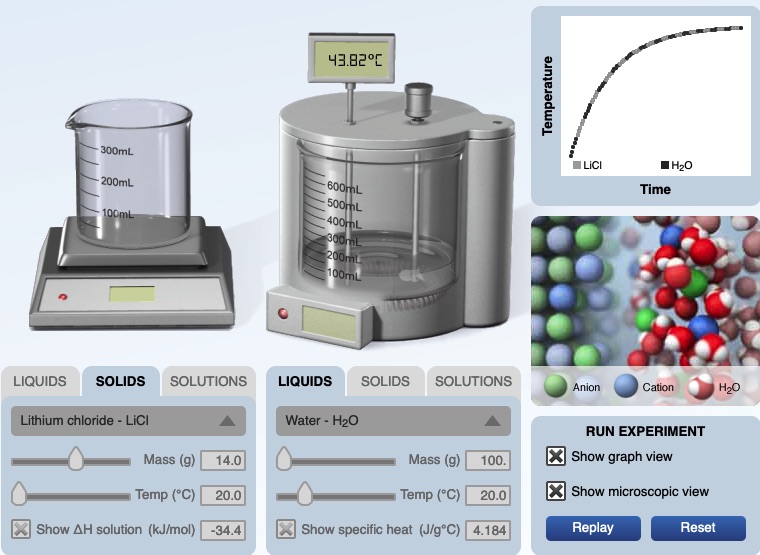
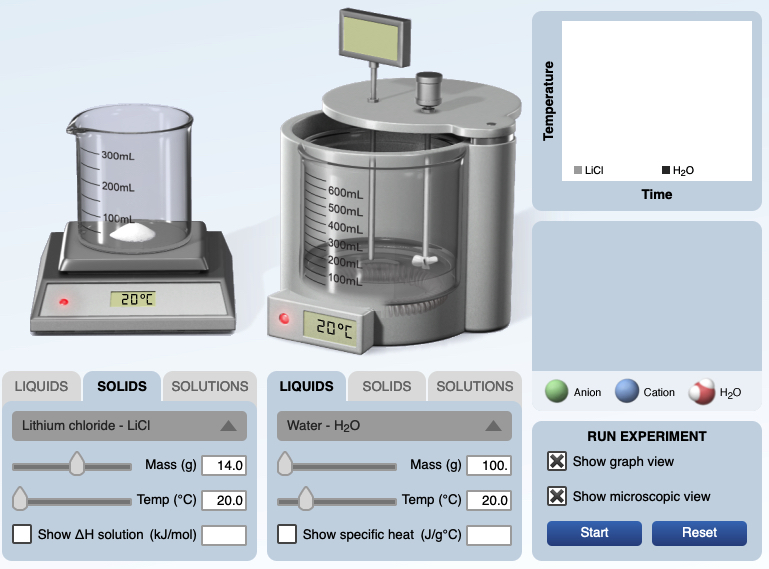
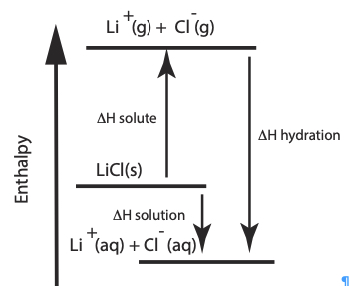
Summary: This page lists components for teachers to build a module on solution formation. The module consists of big ideas, learning objectives, pre-requisite knowledge, computer animations, hands-on calorimetry experiments to determine the change in enthalpy in various solutions (heat of dissolution), computer simulations to determine the change in enthalpy in various solutions, and a theoretical method determine the change in enthalpy in various solutions. This theoretical method involves lattice energy, heat of hydration and energy diagrams. The change in enthalpy value for each method is compared. Animations at the particle level and particle diagrams are used to represent (convey, visualization) what occurs at the particle level (ions and water molecules) when solids dissolve in water. Dynamic animations are used to represent how energy is transferred at the particle level. For exothermic dissolving processes the heat of hydration > lattice energy. The resultant solution gains energy which is manifested in more rapid movement of water molecules and ions - the resultant solution becomes warmer. For endothermic dissolving processes, lattice energy > heat of hydration. The resultant solution releases energy which is manifested in less movement of water molecules and ions - the resultant solution becomes cooler.
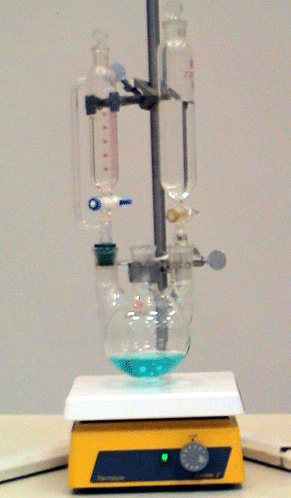
Solution Energetics: Heat of Solution Calorimetry Using Demonstrations, Simulations, Animations, Enthalpy Diagrams
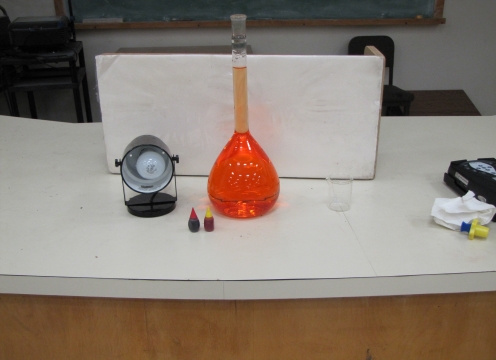
This presentation/lesson incorporates a combination of resources: the use of a calorimeter in which ionic salts dissolve in water (demonstration), a calorimeter computer simula