This presentation/lesson incorporates a combination of resources: the use of a calorimeter in which ionic salts dissolve in water (demonstration), a calorimeter computer simulation, molecular models, animations, and enthalpy diagrams of the dissolving process. The energy changes associated with dissolving two salts are discussed. Lithium chloride, when dissolved in water, will generate a warm solution. The dissolving process for LiCl is an exothermic process because the steps involved in forming the solution releases more energy than it takes in. A second salt, ammonium chloride, when dissolved in water, will generate a cool solution. This dissolving process is an endothermic process because the steps involved in forming the solution absorbs more energy than it releases.
The purpose of the presentation/lesson is to point to resources for chemistry instructors to help students understand several concepts associated with the energy released or absorbed by the process of dissolving a salt in water. While lithium chloride and ammonium chloride are the two salts selected for investigation, there are a variety of soluble ionic salts that may be used. Principles of Universal Design for learning are incorporated in that multiple representations are used. Students are able to make connections among the macroscopic, particle level, and symbolic levels of representation (Johnstone, 1982, 1991, 1993) associated with the dissolving process. An emphasis is placed on particle movement to model the exchange of kinetic energy and we keep track of the changes of potential energy and kinetic energy.
This web page is for chemistry instructors.
"A solution is formed when one substance disperses uniformly throughout another. The ability of a substance to form a solution depends upon two factors: (1) the natural tendency of substances to mix and spread into larger volumes when not restrained in some way, and (2) the types of intermolecular interactions involved in the solution process." (Brown, LeMay, Bursten, et al. Chemistry: The Central Science 13th ed. 2015. Pearson: Hoboken, NJ.).
Main learning objectives: Describing the various steps of energy transfers associated with the dissolving process is one focus of this presentation. Describing how energy is transferred during the dissolving process at the particle level, including explanations of changes in potential energy and kinetic energy, and including explanations of enthalpy changes and entropy changes is another focus. The determination of the change in enthalpy of solution (heat of solution), ∆H solution is discussed using two methods:
1) calorimetry with measurements of mass of the solute and solvent, and the initial and final temperature of the solution. The specific heat of the solution must be known.
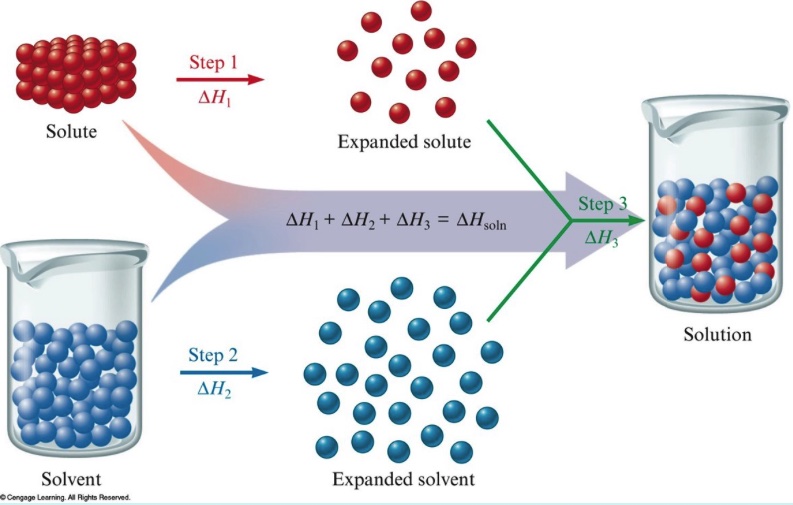
2) qualitative representation of ∆H solution using a particle level view ∆H solution = ∆H solute + ∆H solvent + ∆H mix
calculation using lattice energies, ∆H lattice, and heats of hydration, ∆H hydration, for ionic salts.
We can combine ∆H solvent and ∆H mix and this will equal ∆H hydration.
∆H hydration = ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H hydration
- When an ionic salt dissolves in water, explain the three theoretical steps involved.
- Explain the difference between an exothermic and an endothermic dissolving process.
- Use both kinetic energy and potential energy to explain energy exchanges associated with the dissolving process.
- Draw diagrams, at the particle level, of the steps involved in dissolving.
- Use particle diagrams or animations to illustrate how energy is transferred during the dissolving process.
- Use enthalpy diagrams and particle diagrams to represent the dissolving processes.
- Use information from a calorimetry experiment to determine the change in enthalpy of solution, ∆H solution, for dissolving a salt in water.
- Use lattice energies, ∆H lattice, and heats of hydration, ∆H hydration for ionic salts to determine the change in enthalpy of solution, ∆H solution, for dissolving a salt in water.
This page is under construction. Authors of this web page: T. J. Greenbowe, University of Oregon USA; M. L. DeWane, University of Idaho USA; and Robert Bucat, University of Western Australia.
A. Dissolving lithium chloride in water and dissolving potassium chloride in water: a thermochemistry demonstration
We recommend starting the lesson with a thermochemistry lecture demonstration of dissolving lithium chloride, LiCl, in water and dissolving potassium chloride, KCl, in water using a thermometer to measure the initial and final temperature of the solutions. There are several ways an instructor might choose to present the demonstration. We are presenting two ways. See the links below for examples of how an instructor might present this demonstration.
In the first presentation, an instructor from Pima Community College Arizona choose to present a didactic lecture demonstration. We make no judgements as to how instructors choose to present demonstrations. At the end of the demonstration, thermometers are used to measure the temperature of the resultant solutions. The demonstration, "heats of reactions: a chemical hot and cold pack demonstration" is presented by a chemistry instructor at Pima Community College, Tucson, Arizona (2020).
https://www.youtube.com/watch?v=CKNG7kHO488 [accessed April, 2023]
In the second presentation an instructor presents the demonstration using active learning with a guided-inquiry approach. We hope this approach sparks your interest.
insert description here
insert URL here
For instructors: The two presentations use the same set of chemicals and science equipment. How are the two presentations different? Identify two concepts presented in each presentation. For each presentation, identify one factor that makes the presentation effective. Which presentation matches your typical presentation? Explain.
Calorimetry Computer Simulation
We recommend the demonstration be paired with the "Calorimeter Simulation". The following is an image from the computer simulation experiment for dissolving lithium chloride in water. Before mixing, the temperature of both the solid lithium chloride and water is 20.00°C. After dissolving the temperature of the solution is 43.82°C. A graph of temperature vs. time shows an increase in temperature of the solution.
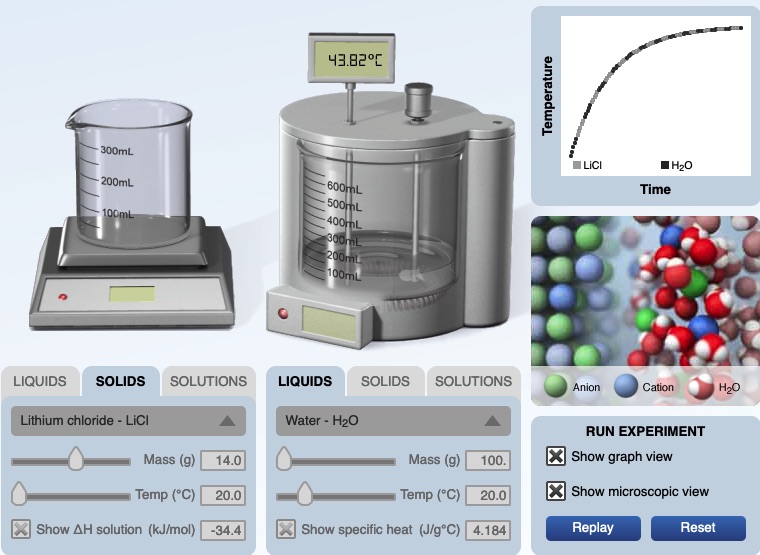
The HTML5 calorimetry computer simulation is available at the following URL. If you use the simulation, please cite the simulation. ©2017 Greenbowe, T., Abraham, M., Gelder, J., Calorimetry Computer Simulation, Pearson: Hoboken, NJ.
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
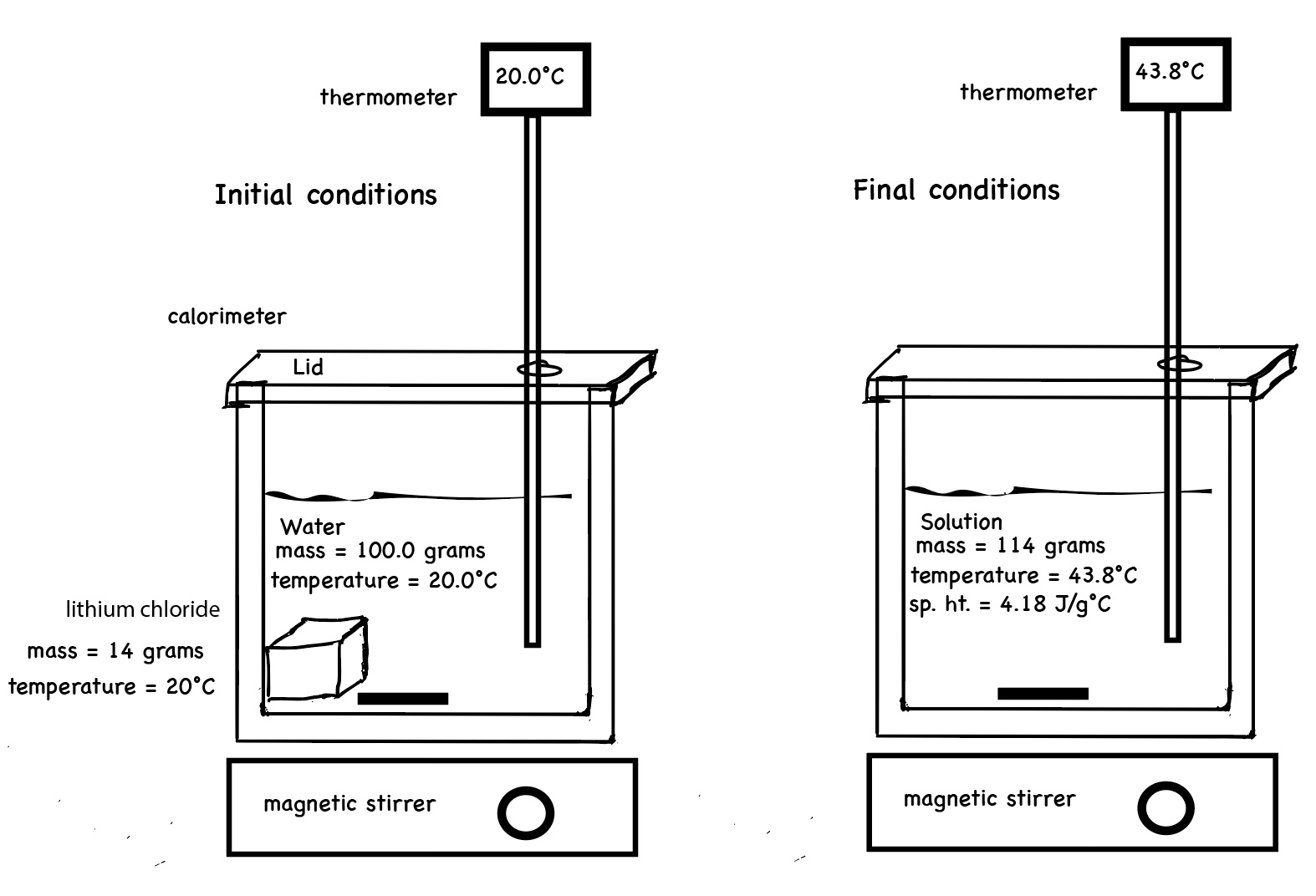
The following two diagrams attempt to illustrate the initial conditions of a calorimetry experiment - dissolving solid lithium chloride in water. Both the solid and the water start at room temperature. The solid salt is added, the water is stirred, the salt dissolves. The resultant solution has a temperature of 43.8°C.
Computer Simulation
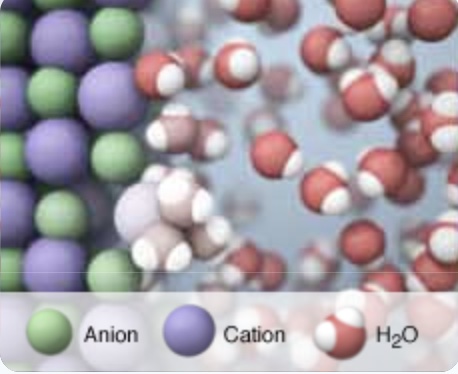
The calorimeter computer simulation has an animation of a particle view (molecular scene) of the interaction of water molecules with the ions in the solid and in the solution during the dissolving process. This particle visualization of the dissolving process is a model of energy transfer. As with any model it has limitations and does not capture all of the complexities of the dissolving process. This model focuses on the changes in kinetic energy of particles and does not explicitly include the contribution of potential energy to the process.
An animation illustrating energy transfer at the particle level of representation for an exothermic dissolving process. This is a short segment from the simulation. Initially, the cations and anions in the solid are vibrating. The cations and anions are held together by electrostatic forces of attractions. An input of energy is required to separate unlike charged particles. Initially, the water molecules are moving about with moderate movement. When water molecules collide with the cations and anions on the surface of the solid, ion-dipole intermolecular forces form (energy is released). The ions become hydrated. Some of this energy goes into separating cations from anions. Some of the energy goes into separating water molecules. Some of the released energy is absorbed by the water molecules and ions. The water molecules and ions have more kinetic energy and move faster. The water molecules and ions hit the thermometer with more impact and the thermometer indicates a higher temperature for the solution. When LiCl dissolves in water, net energy is released during the dissolving process. <= Currently, Firefox 111.0.1 is doing the best at playing the embedded animations (April, 2023). |
Overview
Solid lithium chloride dissolves in water producing a solution containing a 1:1 ratio of hydrated lithium cations and hydrated chloride anions. For example, if 0.050 mole of LiCl dissolves it will generate 0.050 mole of hydrated Li+ ions and 0.050 mole of hydrated Cl- ions, equal number of cations and anions. We can represent this dissolving process by a balanced chemical equation.
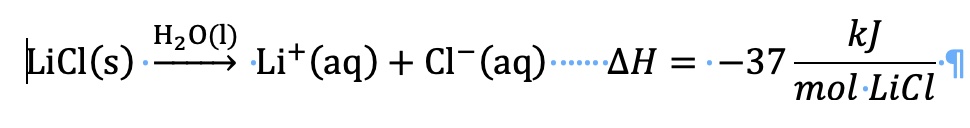
Dissolving an ionic salt in water is a complex process. For the purpose of this presentation, we will assume that the dissolving process has aspects of both a chemical process (intermolecular forces are disrupted, energy is absorbed in order to pull apart ions in a lattice, ion-dipole interactions form, new chemical species form, there is a release or absorption of energy, and in most cases there is a change in the pH of the solution) and a physical process (evaporating the solution leads to recovery of the original salt). The dissolving process involves bond breaking, disruption of hydrogen bonding, and formation of ion-dipole interactions. Chemical bonds (ion-ion), intermolecular forces, and interactions have no mass and have no association with temperature. Breaking and forming chemical bonds involves changing the potential energy of the species involved in the dissolving process (water molecules and ions). A chemical equation has no mass. A chemical reaction has no mass and no temperature. One cannot not directly see a chemical reaction - with respect seeing bonds breaking and bonds forming. The "dissolving process" has no mass and no temperature. One cannot directly see a dissolving process - with respect seeing bonds breaking and bonds forming. We measure the mass, initial and final temperature of the solution. The bulk chemicals - the solid salt and the water - are not the dissolving process. Seeing solid salt disappear into water is an observation and we often say we "see the solid dissolving in the water". However, individuals cannot see the actual "dissolving process". If a dissolving process releases energy, the resultant solution gains this energy. We calculate the thermal energy transferred - absorbed - by the solution, q solution. The thermal energy absorbed by the solution, when LiCl dissolves in water, is a positive quantity.
For this dissolving process, one observes an increase in temperature of the resultant solution. The inference is the solution gained energy. The Law of Conservation of Energy states energy cannot be created or destroyed. Remember, heat is not a type (or form) of energy. In every calorimetry experiment something releases thermal energy and something gains this thermal energy. This is the big idea for calorimetry and must be emphasized to students multiple times. The dissolving process releases the thermal energy that the solution absorbs. If we calculate the thermal energy absorbed by a solution, q solution, we infer the thermal energy released by a dissolving process, q dissolving process, is equal but opposite in sign. This is an application of the Law of Conservation of Energy.
q solution + q dissolving process = 0
Identify What Releases Thermal Energy and What Gains Thermal Energy
It is better to have students first identify what releases thermal energy and what gains thermal energy. After students do an analysis of the experiment, then identify a system and surrounding. When lithium chloride dissolves in water in a calorimeter the dissolving process releases thermal energy and the resultant solution gains thermal energy.
Energy is transferred during a dissolving process from several different sources. There is a net change in enthalpy, ∆H, of the dissolving process.
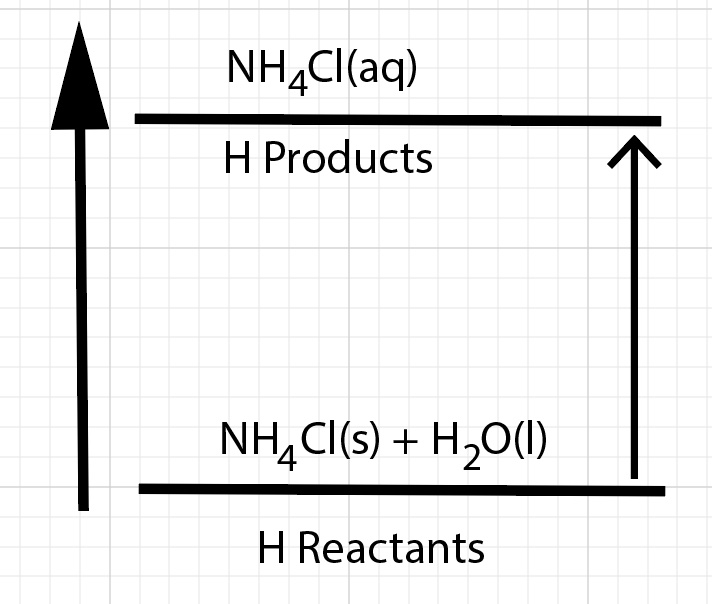
∆Hdissolution = ∆Hsolution = H reactants - H products
For a process that releases energy, the enthalpy of the products are lower compared to the enthalpy of the reactants. The reactants have stronger bonds compared to the products.
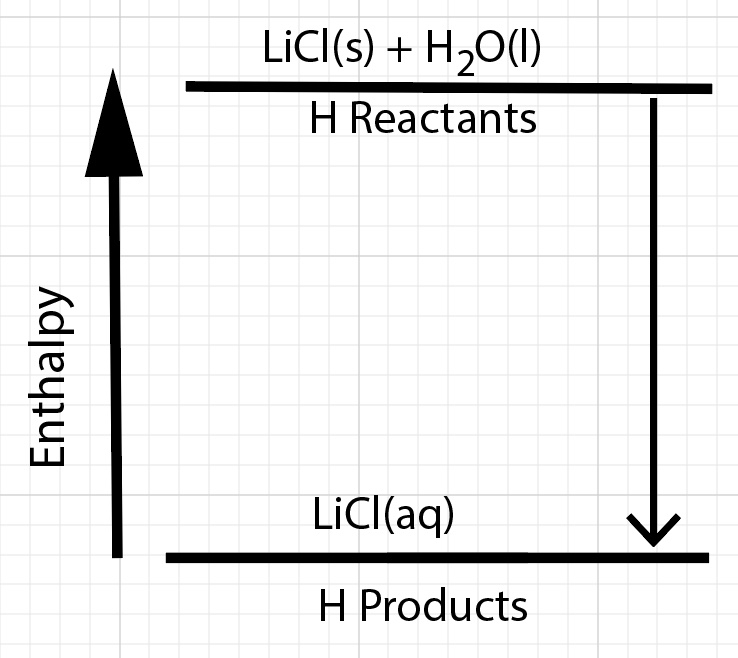
In the case of dissolving solid lithium chloride, the ion-ion interactions of the LiCl in the solid and the hydrogen bonding IMFs of water are stronger compared to the ion-dipole interactions, hydrogen bonding interactions, and ion-ion interactions of ions and molecules in the resultant solution. Bond breaking, bond forming, and ion-dipole interactions forming and breaking are changes in potential energy.
The potential energy released by the dissolution process is transformed (or converted) to an increase in kinetic energy of the molecules and ions in the solution.
Dissolving lithium chloride in water results in a net release of potential energy by the chemical bonds and ion-dipole interactions. Overall, dissolving lithium chloride in water is an exothermic process. By the dissolving process we mean bonds breaking and forming and interactions breaking and forming. The heat of solution, change in enthalpy of solution, ∆Hsolution, is negative. Technically, this is often called the change in enthalpy of dissolution or the heat of dissolution.
Physics
With respect to the physics associated with the dissolving process, there are two main factors to consider - changes in potential energy and changes in kinetic energy. For an exothermic dissolving process there is a net lowering of the potential energy of the "system - defined as bonds and interactions" and there is an overall increase in the kinetic energy of particles (molecules and ions) in the resultant solution. Overall, potential energy is converted (or transformed into) to kinetic energy of vibrating atoms when bonds break. In the case of lithium chloride solid, the ions are vibrating moderately in the solid. When ion-ion forces break, the decrease potential energy is transformed to an increase in kinetic energy of the ions - the ions vibrate vigorously.
For an endothermic dissolving process there is a net raising of the potential energy of the "system" and there is an overall decrease in the kinetic energy of particles (molecules and ions) in the resultant solution. Overall, kinetic energy is converted to potential energy. This will be discussed in the ammonium chloride section.
See Zumdahl and Zumdahl (2014) Chemistry (9th ed.), Cengage: Belmont, CA for a good discussion of the changes in potential energy and kinetic energy associated with chemical reactions.
With respect to the physics associated with the dissolving process, we need to define energy, internal energy, heat, temperature, thermal energy, change in enthalpy and bond dissociation energy. In science, there are a limited number of types of energy: internal energy, potential energy, kinetic energy, chemical energy, mechanical energy, sonic energy, gravitational energy, nuclear energy, electromagnetic energy (x-rays, UV, visible light, IR, radio), and thermal energy. Note, heat is not a type of energy or form of energy!
Energy is defined as the capacity to do work or to "transfer heat" - meaning a process.
Internal energy of a system, E, is the sum of the potential energy and kinetic energy of all particles in the system. A change in the internal energy may be affected by adding or subtracting work, thermal energy, or both. ∆E = q + w
Thermal energy is a type of energy. Thermal energy is the total energy of the moving and or vibrating atoms (kinetic energy), as well as stretching and contracting of bonds (potential energy) inside an object. Atoms and or molecules comprising an object at a specific temperature have thermal energy, but heat cannot be calculated because there is no exchange of heat due to a temperature differential. An object's thermal energy continues to exist when the object is isolated. Thermal energy is proportional to the temperature of an object. However, thermal energy is not temperature. Thermal energy is not heat. For a gas, thermal energy, Eth, can be calculated
Eth = (3/2)nRT for a monatomic gas, such as Helium(g) or Krypton(g)
Heat, q, is the transfer of an amount of thermal energy transferred between two objects at different temperatures or the amount of thermal energy exchanged during a phase change of a pure substance where there is no temperature change (i.e. solid melting to a liquid). Heat is not a substance, not a property of a substance, not a state function, and not a type of energy. Heat is not substance that flows from one object to another. Heat is not measured. The amount of heat exchanged can be calculated.
Temperature is a function of the average translational kinetic energy, Eavg, in a local volume of a sample of gas.
Eavg = (3/2)kBT where kB is a Boltzman constant = 1.38 x 10-23 J/K and T is the temperature in units of Kelvin
T = (2/3kB)Eavg
Temperature is a funcrion of the thermal energy of an object per molecule or per atom. A gas molecule's average translational kinetic energy depends only upon the temperature, not on the molecular mass. The total microscopic kinetic energy is
KE = NAEavg
A thermometer measures the temperature of a local volume in an object. A thermometer does not measure the total energy of an object. Raising the temperature of an object means adding thermal energy sufficient to cause the atoms to vibrate more vigorously (or move faster) and the bonds to stretch and contract more. The amount of thermal energy increases and the temperature increases - hence temperature and thermal energy are related proportionaly.
The Law of Conservation of Energy applied to calorimetry. The total energy of an isolated system (or closed system) is a constant. The kinetic, potential and thermal energy associated with a "system" can be transformed from one to the other, but the sum remains the same. E = KE + PE + TE This is the big idea for all calorimetry experiments.
To better understand the concepts of heat, temperature, and thermal energy it is best to read several physics education articles on these topics and to read a physics textbook.
Thermodynamics
With respect to the thermodynamics associated with the dissolving process, there are two main factors to consider - a change in enthalpy, ∆H, and a change in entropy, ∆S. For every dissolving process there is a change in enthalpy. The change in enthalpy of the dissolving process can overall release energy (an exothermic process) or absorb energy (an endothermic process). For every dissolving process there is an increase in entropy - an increase in microstates - due to a highly ordered array of ions locked in a lattice (order) dispersing throughout a solvent, water, and moving about (disorder).
Conceptual Understanding for an Exothermic Dissolving Process. Ask students: "Where does the energy come from and go to?"
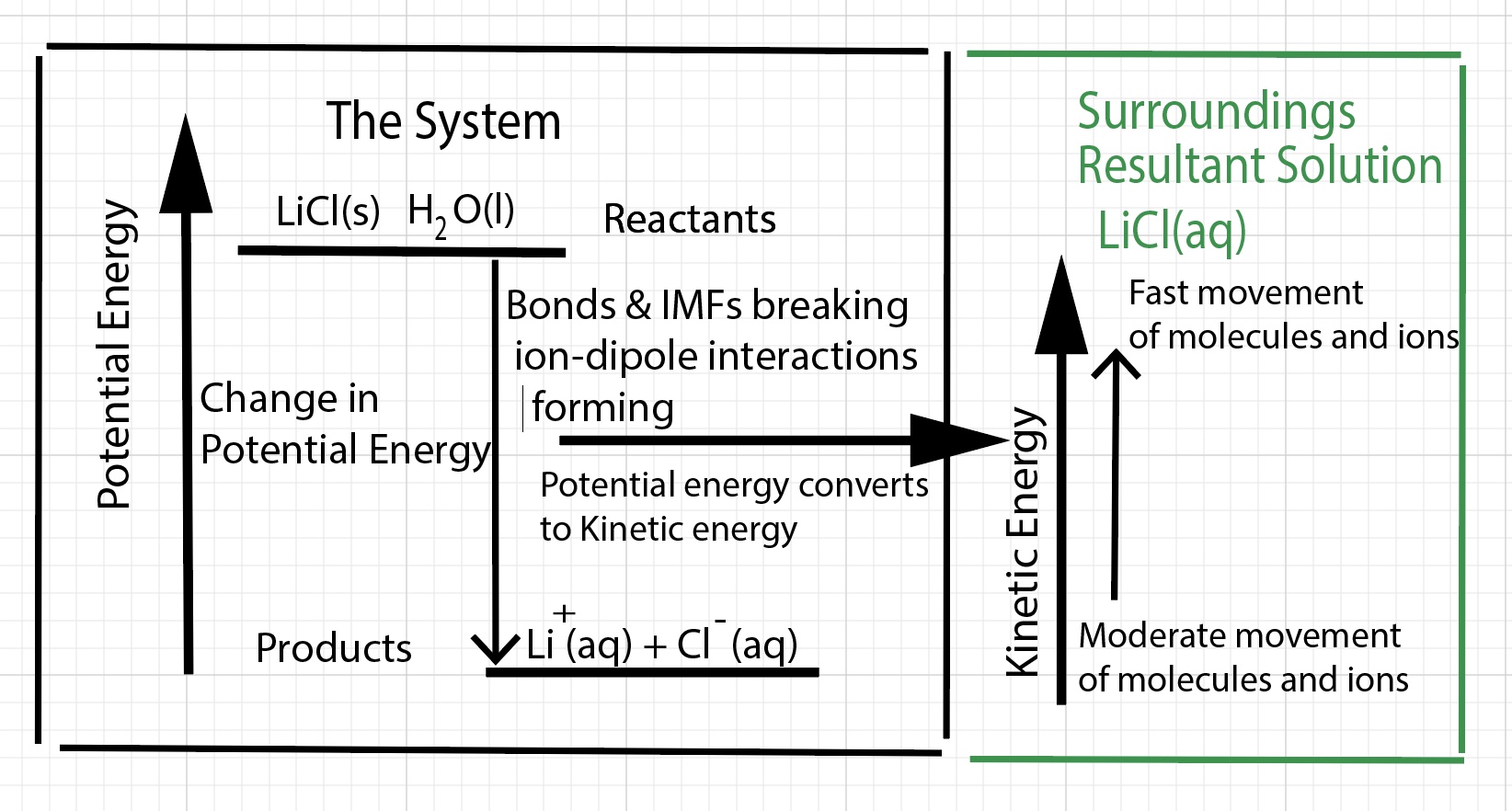
This exothermic dissolving process releases potential energy. The emitted thermal energy does not come from the original thermal energy of the solution. Thermal energy is defined as the total kinetic energy of the atoms and molecules involved in the reaction or process. One indication of what is going on with the particles comes from a measurement of temperature of the solution. When dissolving lithium chloride in water in a calorimeter it is difficult to observe a decrease in temperature of the solution in the initial seconds as the solid is added. The observation is the temperature of the resultant solution (the surroundings) increases. The inferences are 1) the solution gained thermal energy - the kinetic energy of the water molecules and ions increases, 2) the dissolving process (the system) released thermal energy, 3) the potential energy of the system is lowered. Some of the potential energy is converted to thermal energy, and 4) there is an increase in entropy of the system (more microstates).
"The internal energy of a system (the dissolving process) is the sum of its kinetic energy and potential energy."
In an exothermic reaction or process, a big contribution of the net energy released is the change in potential energy (released) when ion-dipole interactions form (heat of hydration) between cations and anions of the salt and water molecules. This release of potential energy is converted into kinetic energy. The water molecules move faster. These faster moving water molecules strike the surface of the solid lattice converting kinetic energy into potential energy. Most of this potential energy goes to breaking ion-ion interactions of the cations and anions (lattice energy) in the solid lattice structure. Some of the faster moving water molecules strike other water molecules. The kinetic energy is converted into potential energy. This potential energy goes into separating water molecules (disrupting hydrogen bonds between water molecules). In an exothermic dissolving process, there is an excess of kinetic energy of the water molecules. The water molecules and ions hit the thermometer with more forceful impact and the thermometer indicates a higher temperature for the solution.
See N. Tro (2023) Chemistry: A Molecular Approach (6th ed), Chapter 7 Section 7.6 page 287, Pearson: Hoboken, NJ. for a good discussion of exothermic and endothermic processes from the molecular view.
| Steps of Dissolution | Kinetic Energy of the solution | Potential Energy of the dissolving process |
| 1. Separating water molecules, ∆H solvent | small decrease --> | small increase |
| 2. Forming ion-dipole interactions, ∆H hydration | large increase | <-- large decrease |
| 3. Separating cations and anions from the solid, ∆H lattice | moderate decrease | --> moderate increase |
Net result for an exothermic dissolving process: Potential Energy is converted to Kinetic Energy | increase | <-- decrease |
In an exothermic reaction, the value of ∆H is negative, energy is released by the dissolving process. Overall, potential energy of the "system" is converted to kinetic energy of the solution. This increase in kinetic energy goes to increasing the movement of water molecules and ions. There is an increase in entropy (∆S). In dissolution, an increase in entropy is a driving force. The formation of aqueous species is also a driving force.
System and Surrounding
A system is a part of the world scientists choose to investigate. For the purpose of this discussion, we assume the calorimeter contains an isolated system as no heat is exchanged outside of the calorimeter. Because the solid salt is poured directly into water this complicates the identification of system and surrounding. We assume the calorimeter has a lid and is a good insulator. One can include a calorimeter correction factor, the heat capacity of the calorimeter, in the calculation. Since we have an excellent calorimeter, no heat enters or leaves the calorimeter. We assume the inside walls of the calorimeter do not add or subtract heat energy from the solution. The world outside the calorimeter is not influenced by the experiments. Chemists look at reactions from the system point of view. If energy is released by the reaction, this is a negative value.
Chemists often define a system as a chemical reaction or a physical process. To be specific, a system can be defined as the reactants and products of a chemical reaction. However, by reactants and products some chemists mean the bond breaking and bond forming process (no mass), not the bulk chemicals (with mass). In the case of dissolving lithium chloride, we define the system as the "dissolving process", the change in bonds and intermolecular forces associated with the reactants and products. We cannot directly measure anything about the dissolving process. We must infer what the dissolving process did by what happens to the solution. The dissolving process consists of a series of steps at the particle level involving breaking bonds and interactions and forming bonds and interactions. Bonds, intermolecular forces, and interactions do not have mass nor do these entities have a temperature that can be measured.
Chemists often define the surroundings as the calorimeter, the experimenter, the room in which the experiment occurs, and everything beyond the room. In the case of dissolving lithium chloride in a calorimeter with an isolated system, surroundings are defined as the resultant solution. The solution has a total mass. The solution consists of the mass of water and the mass of the chemicals - in this case LiCl - lithium ions, Li+, and chloride ions, Cl-. One cannot ignore the mass of the LiCl. The solution has an initial and final temperature, which can be measured. The change in temperature of the solution can be calculated. During the dissolving process the water, the solvent, increases in temperature and due to thermal equilibrium, the bulk chemicals, which are the solute, in the solution also increase in temperature. The solution has a specific heat capacity. The thermal energy absorbed or released by the solution can be calculated using the total mass of the solution, the specific heat capacity of the solution and the change in temperature of the solution.
In the case of dissolving LiCl in water, the resultant LiCl solution increased in temperature. A thermometer measures the temperature of the solution which consists mostly of water molecules and some cations and anions. We infer that the LiCl(aq) solution gained thermal energy (an endothermic process) and that the dissolving process released this thermal energy (an exothermic process).
Alternate experimental set-up and alternate identification of system and surrounding
One can conduct a calorimeter experiment using a bomb calorimeter. In this experiment, the solid salt and water are confined to a small, sealed metal container. The sealed metal container is placed in a water bath. The temperature of the water bath is measured using a thermometer. See diagram. By some internal mechanism the salt, LiCl, is poured into the water in the metal container. This LiCl "dissolving process" releases energy. The metal container absorbs this energy and there is an increase, in the kinetic energy of the atoms of the container and this results in an increase in temperature of the metal. There is an exchange of thermal energy between the atoms of metal container and the water molecules in the water bath. The temperature of the water bath increases.
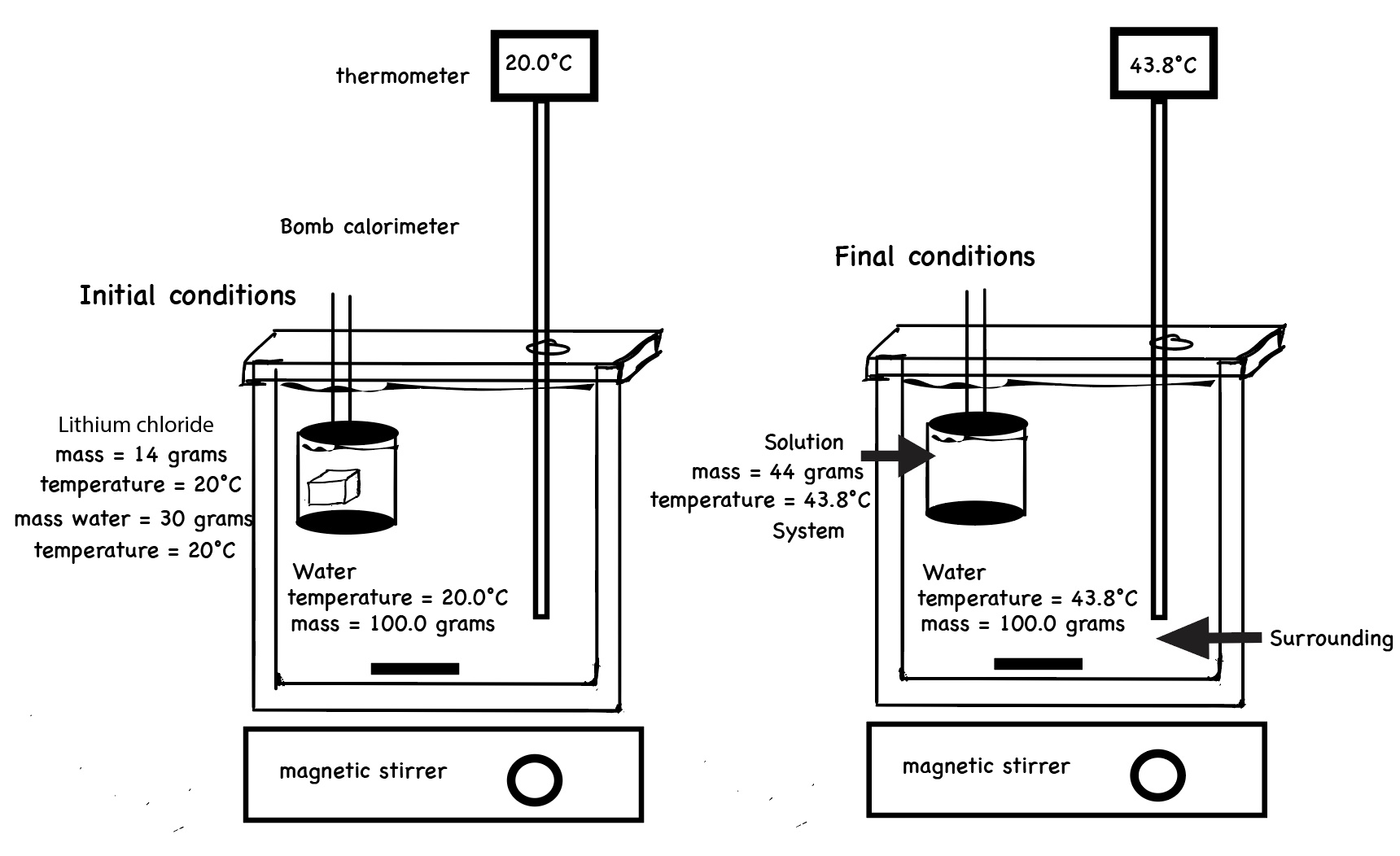
In this experiment, one can identify the system as the bulk chemicals (salt and water) and the surroundings as the water bath. One can measure the mass of the water in the water bath and measure the initial and final temperature of the water bath. The heat energy absorbed by the water bath can be calculated q water bath = mass x specific heat x ∆T
The thermal energy released by the "dissolving process" must be inferred from what occurs in the water bath. In this experiment, there are three components that exchange energy.
The "dissolving process" decreases in potential energy (bonds break and bonds form). This potential energy is converted to kinetic energy which is released as kinetic energy to the atoms in the metal container. The atoms of the metal container release kinetic energy to the water molecules in the water bath.
What releases thermal energy and what gains thermal energy? The net effect is the "dissolving process" releases energy, and the water bath absorbs energy. The thermal energy released by the "dissolving process", q dissolving process, can be calculated
q water bath + q dissolving process = 0
Steps of Dissolution and the Energy Associated with Each Step
1. Separation of solvent molecules. Hydrogen bonds, dipole-dipole forces and dispersion forces are present between water molecules. An input of energy is required to separate water molecules. This is an endothermic process - energy is absorbed by the water molecules to disrupt intermolecular forces present between water molecules. The kinetic energy of the water molecules increase - the molecules move faster.
2. Hydration of ions. Cations and anions form ion-dipole interactions with water molecules. The formation of this ion-dipole interaction (some say an IMF) releases energy in the process. Hydration is an exothermic process.
3. Separation of cations and anions in the solid. The energy released by hydration is absorbed by the cations and anions. The cations and anions move, and this movement helps to overcome the electrostatic forces present between cations and anions in the lattice structure of the ionic compound. This is an endothermic process. The cations and anions are pulled out of the lattice by water molecules.
B. Dissolving Ammonium Chloride in Water, an Endothermic Process
Start this portion of the presentation with a chemistry lecture demonstration of dissolving ammonium nitrate in water. The following video illustrates one way of doing this demonstration.
Video courtesy of BerkeleyChemDemos. University of California Berkeley College of Chemistry Demonstrations Laboratory. B17 Pimentel Hall University of California Berkeley, CA 94720-1460
Solid ammonium chloride dissolves in water producing a solution containing a 1:1 ratio of hydrated ammonium cations and hydrated chloride anions. For example, if 0.050 mole of ammonium chloride dissolves, 0.050 mole of hydrated NH4+ ions form and 0.050 mole of hydrated Cl- ions form, equal number of cations and anions. We can represent this process by a chemical equation.
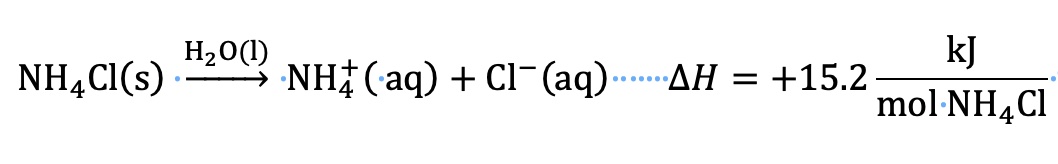
Please see the posted information about this demonstration on the CIDER web site.
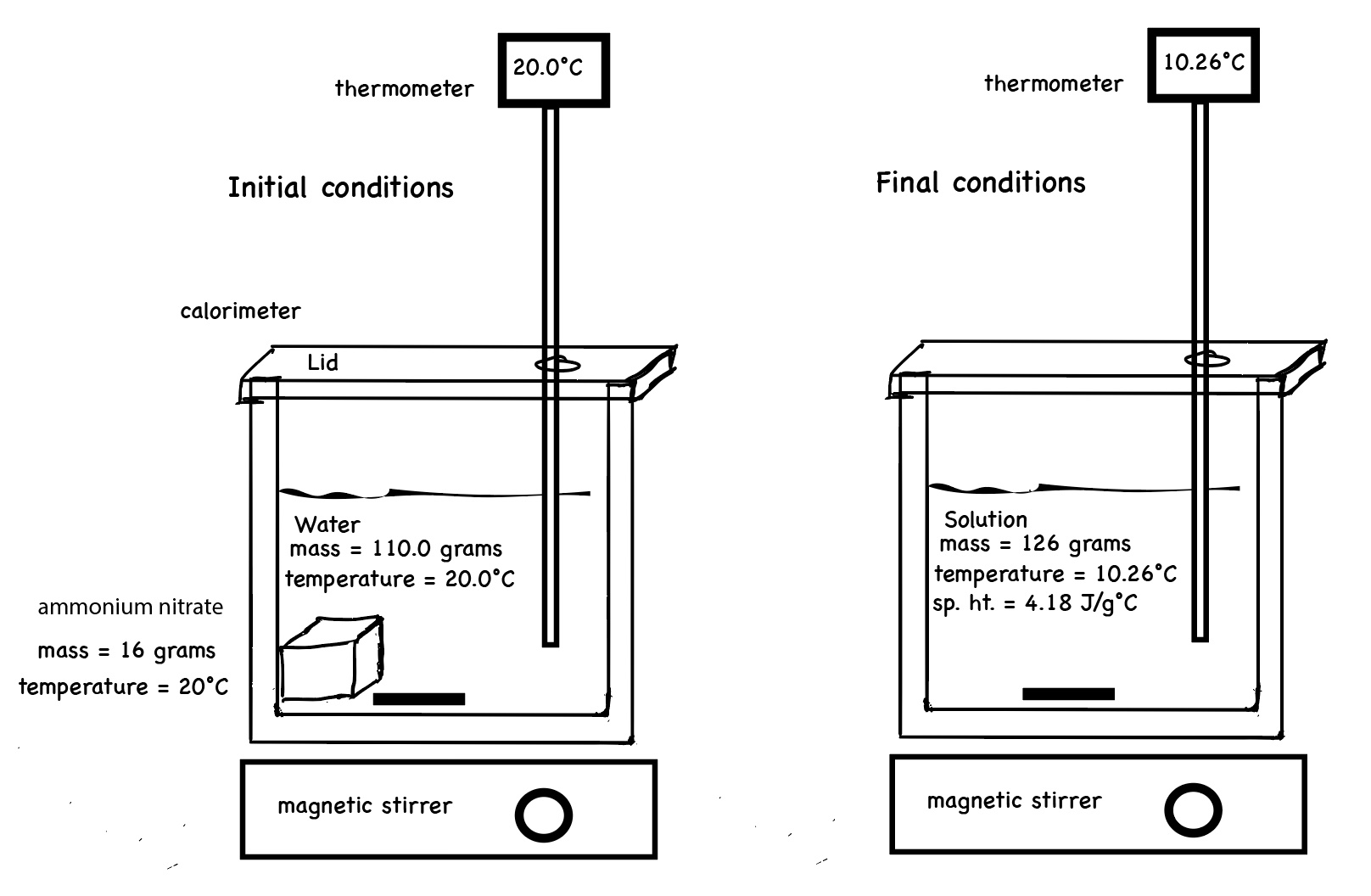
Computer simulation experiment for dissolving ammonium nitrate in water. The solid ammonium nitrate and water have an initial temperature of 20.00°C. After dissolving the temperature of the solution is 10.26°C. A temperature versus time graph shows a decrease in temperature of the solution over time.
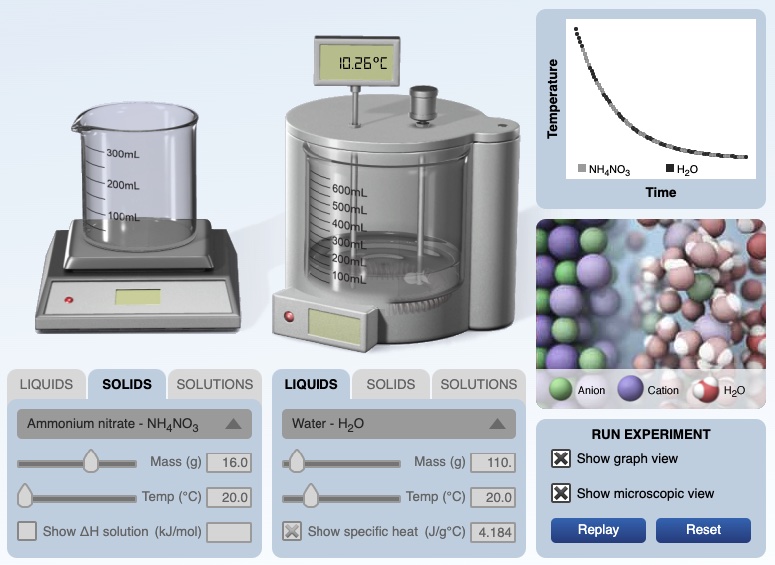
The HTML5 calorimetry computer simulation is available at the following URL. If you use the simulation, please cite the simulation. ©2016 Greenbowe, T., Abraham, M., Gelder, J. Pearson: Hoboken, NJ. Teachers may not use the simulation in any instructional materials sold for profit.
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
Identify What Releases Thermal Energy and What Gains Thermal Energy
What releases thermal energy and what gains thermal energy? When ammonium chloride dissolves in water the resultant solution releases thermal energy and the dissolving process absorbs thermal energy.
Energy is transferred during a dissolving process from several different sources. As a result of the dissolution process, there is a change in enthalpy of the solution. Dissolving ammonium chloride in water results in a net absorbance of energy by the chemical bonds and ion-dipole interactions. The products have greater enthalpy compared to the reactants. Overall, dissolving ammonium chloride in water is an endothermic process. The heat of solution, change in enthalpy of solution, ∆Hsolution, is positive. Technically, this is often called the change in enthalpy of dissolution or the heat of dissolution.
Physics
With respect to the physics associated with the dissolving process, there are two main factors to consider - changes in potential energy and changes in kinetic energy. For an endothermic dissolving process there is a net raising of the potential energy of the "system - defined as bonds and interactions" and there is an overall decrease in the kinetic energy of particles (molecules and ions) in the resultant solution. Overall, kinetic energy is converted to potential energy. For an endothermic dissolving process there is a net raising of the potential energy of the "system" and there is an overall decrease in the kinetic energy of particles (molecules and ions) in the resultant solution. Overall, kinetic energy is converted to potential energy
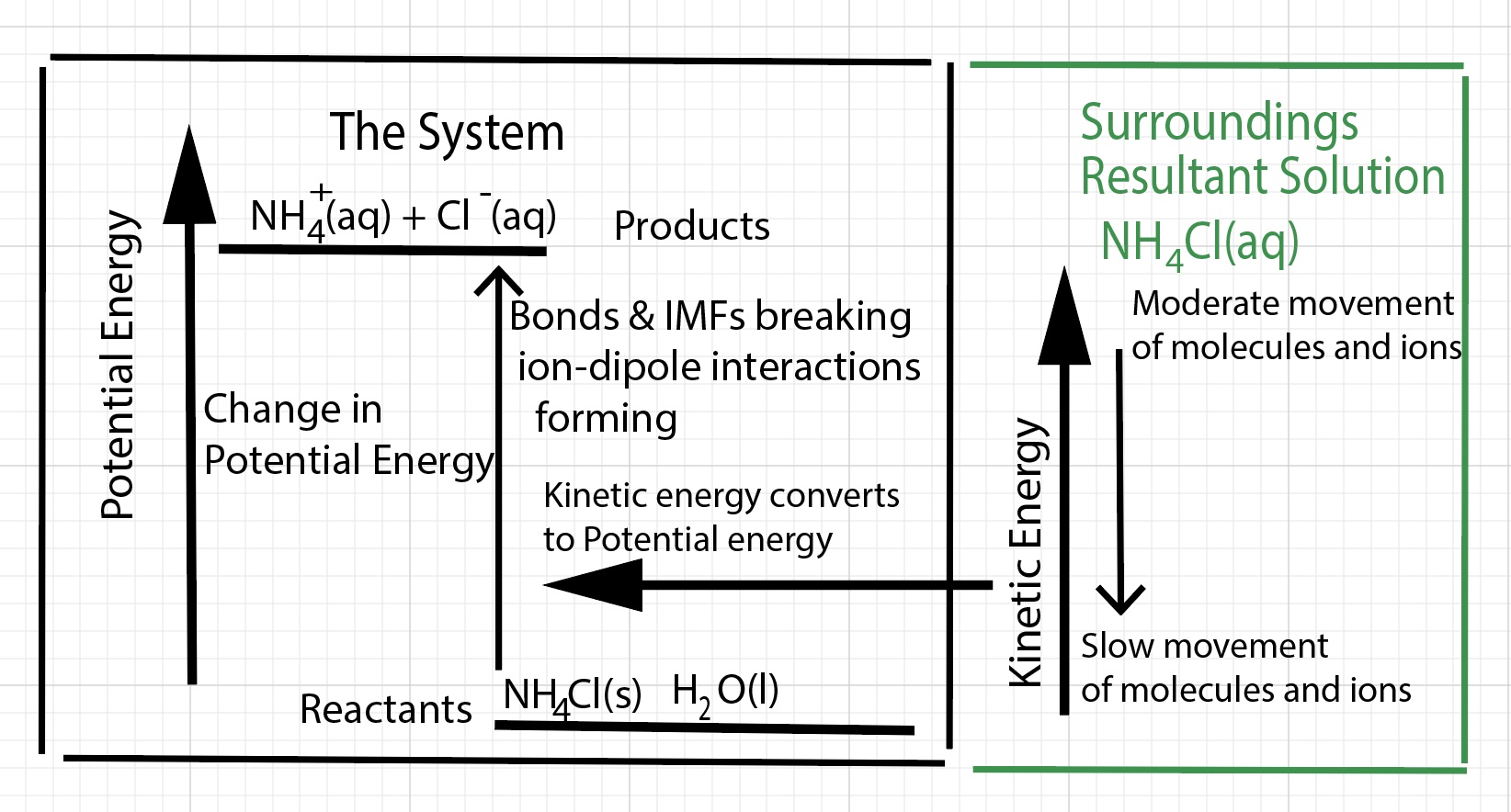
Thermal energy (kinetic energy) is taken away from the solution is converted to potential energy and absorbed by the dissolving process. Most of the energy absorbed goes into separating cations from anions in the solid (breaking ion-ion interactions, lattice energy). Some of the energy goes into separating water molecules (disrupting hydrogen bonds between water molecules). The water molecules have less kinetic energy and slow down. The water molecules hit the thermometer with less impact and the thermometer indicates a lower temperature for the solution.
As a result of the dissolution process, there is a change in enthalpy of the solution. For this endothermic dissolving process, the change in enthalpy of dissolution is positive, energy is absorbed by the dissolution process, an endothermic process. The change in enthalpy of dissolution is called the heat of solution, ∆H solution or the heat of dissolution. The calorimeter computer simulation has an animation of a particle view (molecular scene) of the interaction of water molecules with the ions in the solid during the dissolving process. This particle visualization of the dissolving process is a model of energy transfer that focuses on the kinetic energy of particles. As with any model it has limitations and does not capture all of the complexities of the dissolving process. This model does not explicitly include a visualization of the changes in potential energy.
An animation illustrating energy transfer at the particle level of representation for an endothermic dissolving process. This is a short segment from the simulation. Initially, cations and anions in the solid are vibrating moderately. The cations and anions in the solid are held together by electrostatic forces. An input of energy is required to separate unlike charged particles. When water molecules collide with the cations and anions on the surface of the solid, ion-dipole intermolecular forces form (energy is released). The cations and anions become hydrated (surrounded by water molecules). This releases energy. Most of the energy released by hydration goes into separating cations from anions in the solid. Some of the energy goes into separating water molecules. The dissolution for ammonium chloride requires a significant input of energy - more so than the energy released by hydration. Kinetic energy is transferred from the water molecules to the ions in the solid. The water molecules and ions have less kinetic energy and slow down. The water molecules and ions in solution hit the thermometer with less impact and the thermometer indicates a lower temperature for the solution. When NH4Cl dissolves in water, net energy is absorbed by the dissolving process. <= Currently, Firefox 111.0.1 is doing the best at playing the embedded animations (April 2023). |
Conceptual Understanding for an Endothermic Dissolving Process. Ask students: "Where does the energy come from and go to?"
This endothermic dissolving process absorbs thermal energy. Some of the absorbed thermal energy does come from the original thermal energy of the surroundings"- the resultant solution, mostly water molecules. Thermal energy is defined as the total kinetic energy of the atoms and molecules involved in the reaction or process. One indication of what is going on with the particles comes from a measurement of temperature of the solution. When dissolving ammonium chloride in water the observation is the temperature of the resultant solution (the surroundings) decreases. The inferences are 1) the solution released thermal energy - the kinetic energy of the water molecules and ions decrease. 2) thermal energy is converted to potential energy. 3) the dissolving process (the system) absorbs potential energy, the potential energy of the system is raised. 4) most of this potential energy goes into breaking ion-ion interactions, and 5) there is an increase in entropy of the system (more microstates).
"The internal energy of a system (the dissolving process) is the sum of its kinetic energy and potential energy." In an endothermic reaction or process, when ion-dipole interactions form between cations and anions of the salt and water molecules there is a release of potential energy (heat of hydration). This potential energy is converted to kinetic energy. The water molecules surrounding the ions move faster (vibrate more vigorously).
This kinetic energy is absorbed by the system (in this case the dissolving process) as potential energy and goes into breaking ion-ion interactions of the cations and anions in the solid lattice structure. The potential energy of the system increases. In this process, the water molecules and ions move slower. In an endothermic dissolving process, there is a net loss of kinetic energy of the water molecules. The water molecules and ions hit the thermometer with a less forceful impact and the thermometer indicates a lower temperature for the solution.
See N. Tro (2023) Chemistry: A Molecular Approach (6th ed), Chapter 7 Section 7.6 page 287, Pearson: Hoboken, NJ. for a good discussion of exothermic and endothermic processes from the molecular view.
Steps of Dissolution and the Energy Associated with each Step in an Endothermic Dissolving Process
1. Separation of solvent molecules. Hydrogen bonds, dipole-dipole forces and dispersion forces are present between water molecules. An input of energy is required to separate water molecules. Hydrogen bonds between water molecules can be represented as potential energy. Potential energy is absorbed by the water molecules and the result is the disruption of intermolecular forces present between water molecules. This is an endothermic process. Kinetic energy from neighboring water molecules striking other water molecules is the action that transfers energy. The kinetic energy of the striking water molecules decreases- the molecules move slower.
2. Hydration of ions. When cations and anions form ion-dipole interactions with water molecules potential energy is released. The formation of this ion-dipole interaction is called the Heat of Hydration, and this is an exothermic process. This potential energy is converted to kinetic energy, the water molecules surrounding the ions move faster.
3. Separation of cations and anions in the solid. The fast moving water molecules, strike the surface of the solid, some kinetic energy is converted into potential energy. Some of this potential energy is absorbed by the cations and anions in the solid. This goes to break the ion-ion interactions. The cations and anions also gain kinetic energy and vibrate vigorously. This movement helps to overcome the electrostatic forces present between cations and anions in the lattice structure of the ionic compound. This breaking of ion-ion interactions is an endothermic process. The cations and anions form ion-dipole interactions with water molecules and the ions are pulled out of the lattice by water molecules.
The steps for the dissolution of ammonium chloride are the same as for lithium chloride. However, the energy required to separate the cations from the anions is larger. What drives the formation of a solution with an endothermic process?
| Steps of Dissolution | Kinetic Energy of the solution | Potential Energy of the dissolving process |
| 1. Separating water molecules, ∆H solvent | small decrease --> | small increase |
| 2. Forming ion-dipole interactions, ∆H hydration | large increase | <-- large decrease |
| 3. Separating cations and anions from the solid, ∆H lattice | large decrease --> | large increase |
Net result for an endothermic dissolving process: Kinetic Energy is converted to Potential Energy | decrease --> | increase |
In an endothermic reaction, the value of ∆H is positive, energy is absorbed by the dissolving process. This positive ∆H value is overcome by a sufficient increase in entropy (∆S). In dissolution, an increase in entropy is a driving force. The formation of aqueous species is another driving force.
The Role of Entropy in the Dissolving Process
When cations and anions are forced out of a lattice structure and into solution the number of microstates increases. There is an increase in entropy. Mixing leads to a more random distribution of particles.
Solid (ions are in fixed positions, order) --> Solution (ions move about in random fashion, disorder)
"The formation of solutions is favored by the increase in entropy that accompanies mixing." (Brown, LeMay, Bursten, et al. Chemistry: The Central Science 13th ed. 2015. Pearson: Hoboken, NJ.).
Table: Changes in enthalpy and entropy of each stage of dissolution of ionic compounds.
| Steps of Dissolution | ∆H | ∆S |
| 1. Separating water molecules, ∆H solvent | positive, endothermic | positive |
| 2. Forming ion-dipole interactions, ∆H hydration | negative, exothermic | negative |
| 3. Separating cations and anions from the solid, ∆H lattice | positive, endothermic | positive |
- When the heat of hydration of a salt is greater than the heat of solute (lattice energy), the dissolving process is exothermic.
- When the heat of hydration of a salt is less than the heat of solute (lattice energy), the dissolving process is endothermic.
In an endothermic reaction, the value of ∆H is positive, energy is absorbed by the dissolving process. This positive ∆H value is overcome by a sufficient increase in entropy (∆S). In dissolution, an increase in entropy is a driving force. Another driving force is the formation of aqueous species of ions, M+(aq) and X-(aq).
Representing solids, liquids, and solutions using particle diagrams and models. Basic definitions and concepts.
C. Solid LiCl. The ions are held together by strong electrostatic forces.
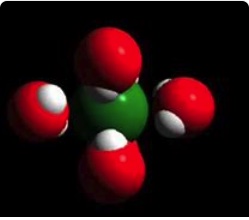
We can represent the solid lithium chloride using particle diagrams. The following diagram represents a view of a small portion of lithium chloride solid, LiCl(s). The small purple spheres represent lithium ions, Li+, and the large green spheres represent chloride ions, Cl-. Understanding how the Li+ ions and Cl- ions are held together by electrostatic forces of attraction and these forces must be overcome (input of energy) in order to dissolve the solid is essential.
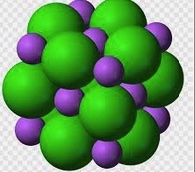 | 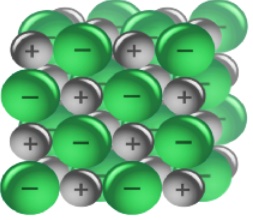 |
Unlike charges attract. The positive lithium ions and negative chloride ions act on each other to create a strong force of attraction. It takes an input of energy to separate cations from anions in a lattice structure.
3D Molecular Design NaCl model https://3dmoleculardesigns.com/product/nacl-lattice/

This model is an effective teaching tool. Have students gently try to separate the small spheres (cations) and the large spheres (anions). Students need to impart some energy in order to break the magnetic force of attraction between the spheres. [Yes, the Na+ ions and Cl- ions are held together by electrostatic forces, but the analogy holds.] Students can bring a Na+ sphere (blue) close to the lattice and it will snap into the lattice with a loud click - representing energy released when a bond forms. Students can bring a Cl- sphere (green) close to the lattice and it will snap into the lattice with a loud click - representing energy released when a bond forms.
Conceptual Understanding: It takes an input of energy to break a bond. When bonds form, energy is released.
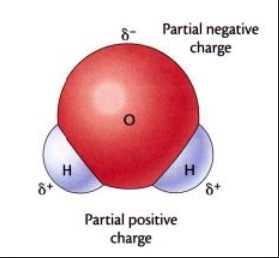
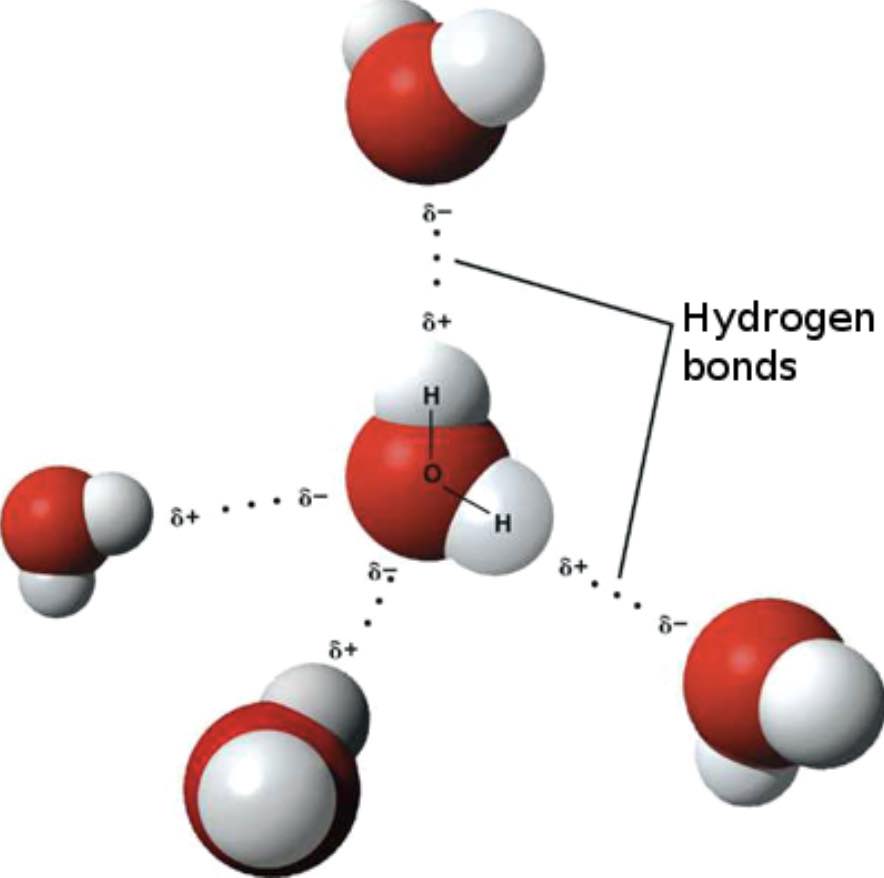
D. Liquid water. Water molecules in liquid water are moving about. Water, H2O, is a polar molecule. There are interactions between and among water molecules in the liquid state called hydrogen bonding (an Intermolecular Force, IMF).
The diagrams below represent an individual water molecule, H2O, and a view of a small portion of liquid water showing groups of water molecules. A large red sphere represents an oxygen atom and the gray-white small spheres represent hydrogen atoms. During the dissolving process, water molecules must pull apart from each other and break hydrogen bonds (IMFs). This requires an input of energy. To separate the cations from the anions in the solid, several water molecules must form ion-dipole interactions in essence attaching to an ion. This releases energy. Water molecule then lift an ion away from other ions. This requires an input of energy. Water molecules pull the ions out of the solid structure and into solution.
 |  |  |
We strongly recommend showing the Roy Tasker animation at the particle level for liquid water. Liquid water Tasker animation
It takes an input of energy to separate water molecules.
Show students the 3D Molecular Design Water molecules. Students have the opportunity to experience a large group of water molecules being held together by hydrogen bonding [in this case by magnetic forces, but it is an analogy that is effective]. https://3dmoleculardesigns.com/classroom_resources/water-kit/

Students experience the force of attraction BETWEEN water molecules. With a modest input of energy these forces between water molecules can be disrupted. The covalent bonds between hydrogen and oxygen atoms do not break.
E. Representing the dissolving process at the particle (molecular) level
Water molecules play a central role in the dissolving process. Water is involved in all three steps of the dissolving process.
- 1) Some water molecules separate from a group of water molecules. This requires an input of energy.
- 2) The water molecules form ion-dipole interactions with the cations and anions in the solid. This releases energy - it is an exothermic process.
- 3) The water molecules drag ions out of the solid and into solution. Some of the released energy goes into separating individual cations and anions from the lattice. Separating a cation from an anion requires an input of energy.
Some of the released energy goes into increasing the kinetic energy of groups of water molecules surrounding the ions. The water molecules and ions move faster.
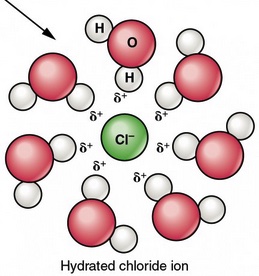
The symbol Li+(aq) means a lithium cation is surrounded by six or seven water molecules in aqueous solution. This is called hydration. The symbol Cl-(aq) means a chloride anion is surrounded by six or seven water molecules in aqueous solution. The water molecules are not fixed around a specific ion. Water molecules detach from an ion-dipole interaction and different water molecules form a new ion-dipole interactions. In solution, on average, six or seven water molecules surround a cation. Six or seven water molecules surround an anion.
The heat of hydration (or the change in enthalpy of hydration) is defined as the enthalpy change for the hydration of one mole of separated gaseous ions.
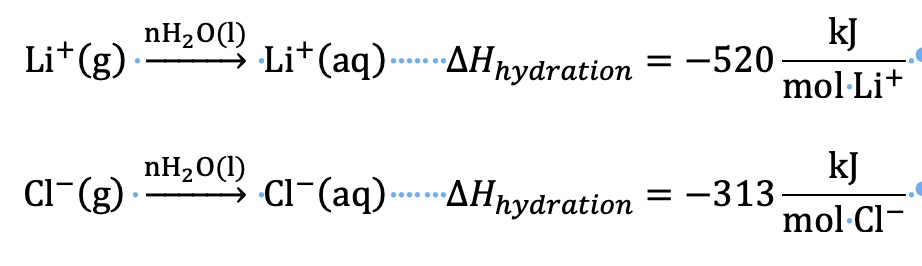
The heat of hydration for ions is determined by doing several physics experiments. During the dissolving process, the cations and anions are not in the gaseous state. We will see how we will be able to make use of these heat of hydration values to calculate the heat of solution.
The water molecules are surrounding an ion are oriented in a specific way. The partial positive charged hydrogen atoms in a water molecule are oriented toward the negatively charge chloride ions. The partial negative charged oxygen end of a water molecule is directed at the positive lithium ion. When ion-dipole IMFs form there is a release of energy, called the heat of hydration or the change in enthalpy of hydration, ∆H hydration.
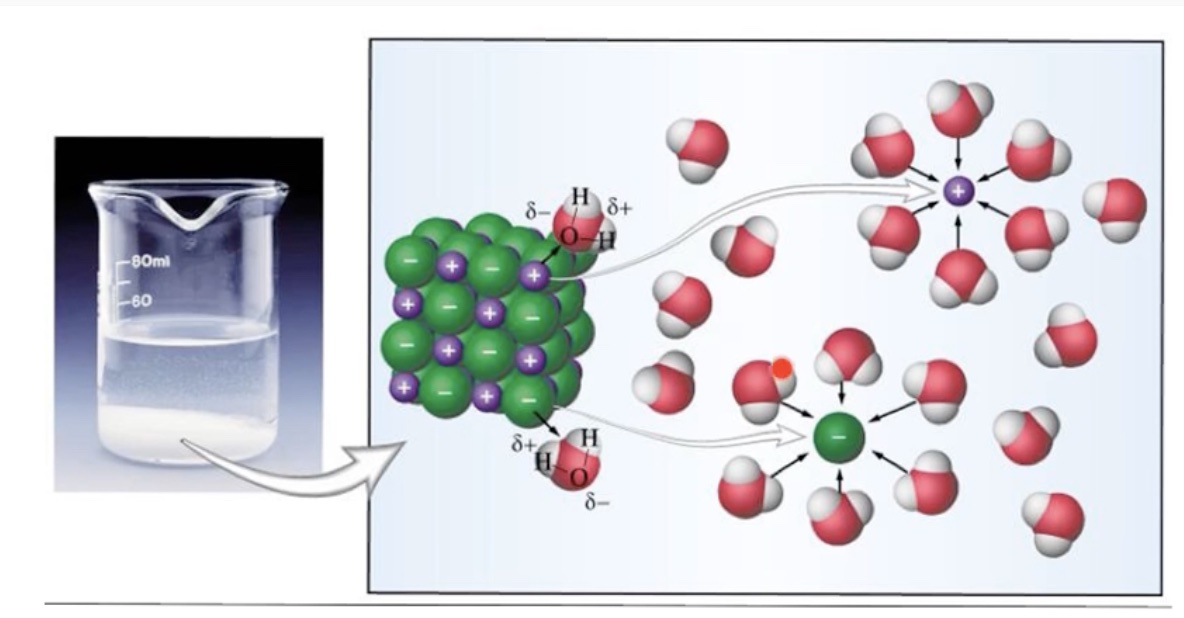
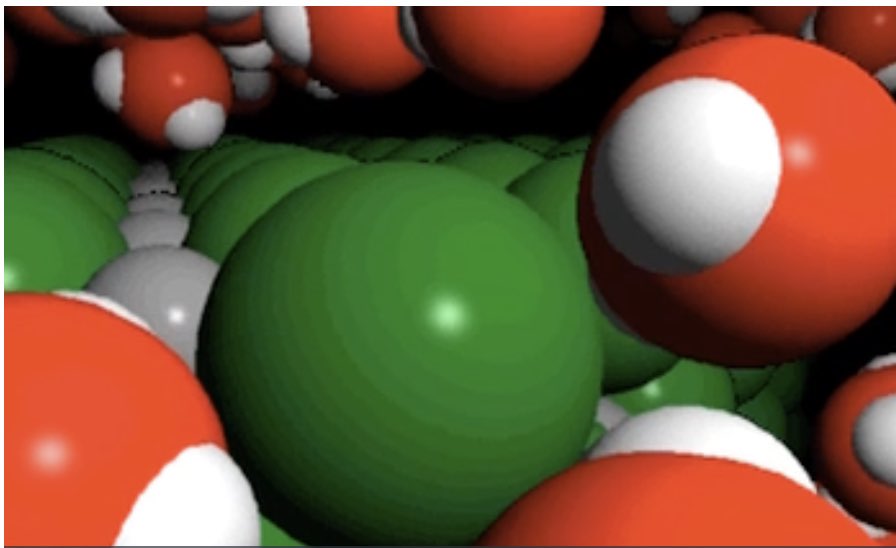
The following space filling model and particle level diagram illustrates how water molecules are oriented around a chloride ion.
 |  |
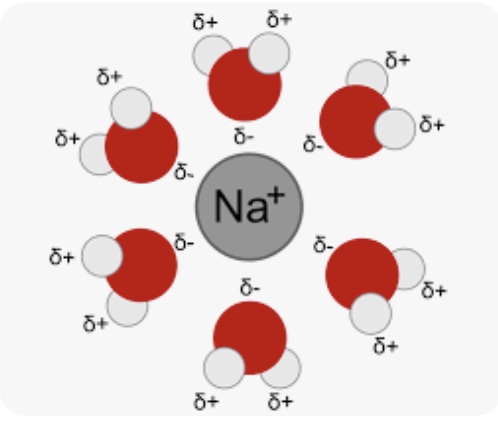
The following particle level diagram illustrates how water molecules are oriented around a sodium ion. A similar arrangement exists for a hydrated lithium ion.
F. Representing the dissolving process of an ionic salt using molecular models and animations
The dissolving process involves water molecules interacting with the lithium cations and chloride anions. Water molecules are polar, having a partial positive end and a partial negative end. Some water molecules orient with their partial positive hydrogen atoms to a negative chloride anion. Other water molecules orient with their partial negative oxygen atoms toward a lithium cation.
Show students the 3D Molecular Design water molecules and the NaCl model combined together to represent ion-dipole IMFs with the two types of water molecule orientations to the ions. The blue spheres can represent either Na+ cations or Li+ cations. The green spheres represent chloride ions, Cl-.

Students experience a force of attraction BETWEEN the water molecules and the chloride ion and BETWEEN the water molecules and the lithium ion (or sodium ion). This is another type of representation for ion-dipole interactions. Students see a three-dimensional model of how water molecules orient around a cation and around an anion. With a modest input of energy, the forces of attraction between water molecules and ions can be disrupted. The covalent bonds between hydrogen and oxygen atoms do not break.
G. Dissolving an ionic salt in water - animation
Dissolving an ionic salt in water is a dynamic process. The following short animations provide a visual representation of the dissolving process. The animations are about dissolving sodium chloride, NaCl. However, the same process occurs for dissolving lithium chloride and for dissolving ammonium chloride.
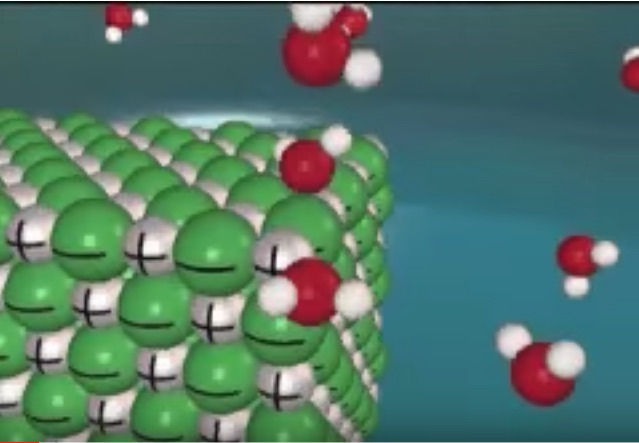
Dissolving an ionic salt in water particle level animation
Tasker image for dissolving NaCl in water. We strongly recommend showing the Roy Tasker animation for dissolving NaCl in water. There is an activity sheet to accompany this lesson.
Dissolving NaCl in water Tasker animation
What did you observe in the animation? There is a student handout for this activity. Dissolving is a dynamic process. This animation focuses on the role of the water molecules.
Ask students, "If dissolving all salts encompasses similar processes, why are some dissolving processes exothermic and some endothermic?"
H. Calorimetry Experiments
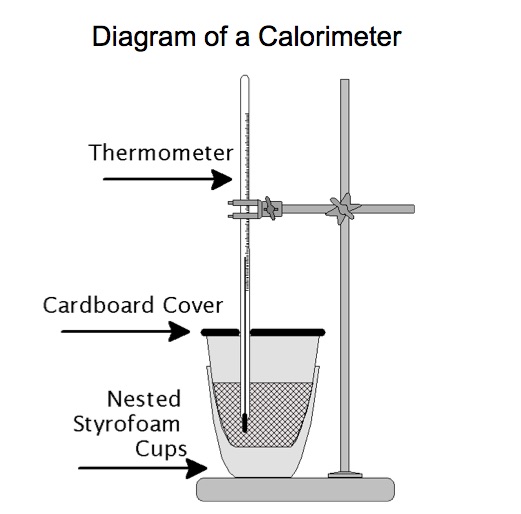
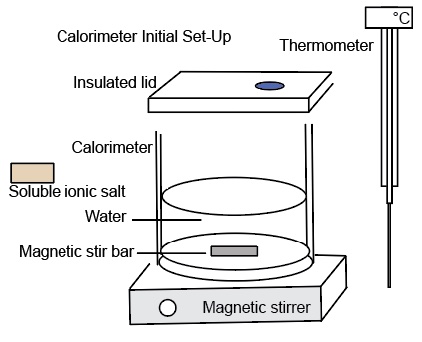
It is useful to have students observe the temperature change that occurs when a salt dissolves in water. We recommend doing a dissolution calorimetry experiment when studying the solution formation chapter. We can do two quick calorimeter "experiments". The calorimetry experiments in this presentation are not designed to be accurate laboratory experiments. For these two experimental set-ups, each salt is placed directly into the water and stirred by a magnetic stirrer.
Measure the initial mass of the salt and the initial mass of the water. Measure the initial temperature of the salt and the initial temperature of the water prior to mixing. Record the masses and the temperatures. Place the water in the calorimeter. Start the computer program and record the temperature of the water as a function of time for about 1 minute. Pour the salt into the water and place a lid on the calorimeter. The digital thermometer measures the temperature of the resultant solution. The computer program records the temperature of the solution as a function of time. We notice the temperature of the solution increases. When all of the salt dissolves and the temperature of the solution remains the same or begins to change, stop the experiment. Determine the change in temperature of the resultant solution.
 |  |
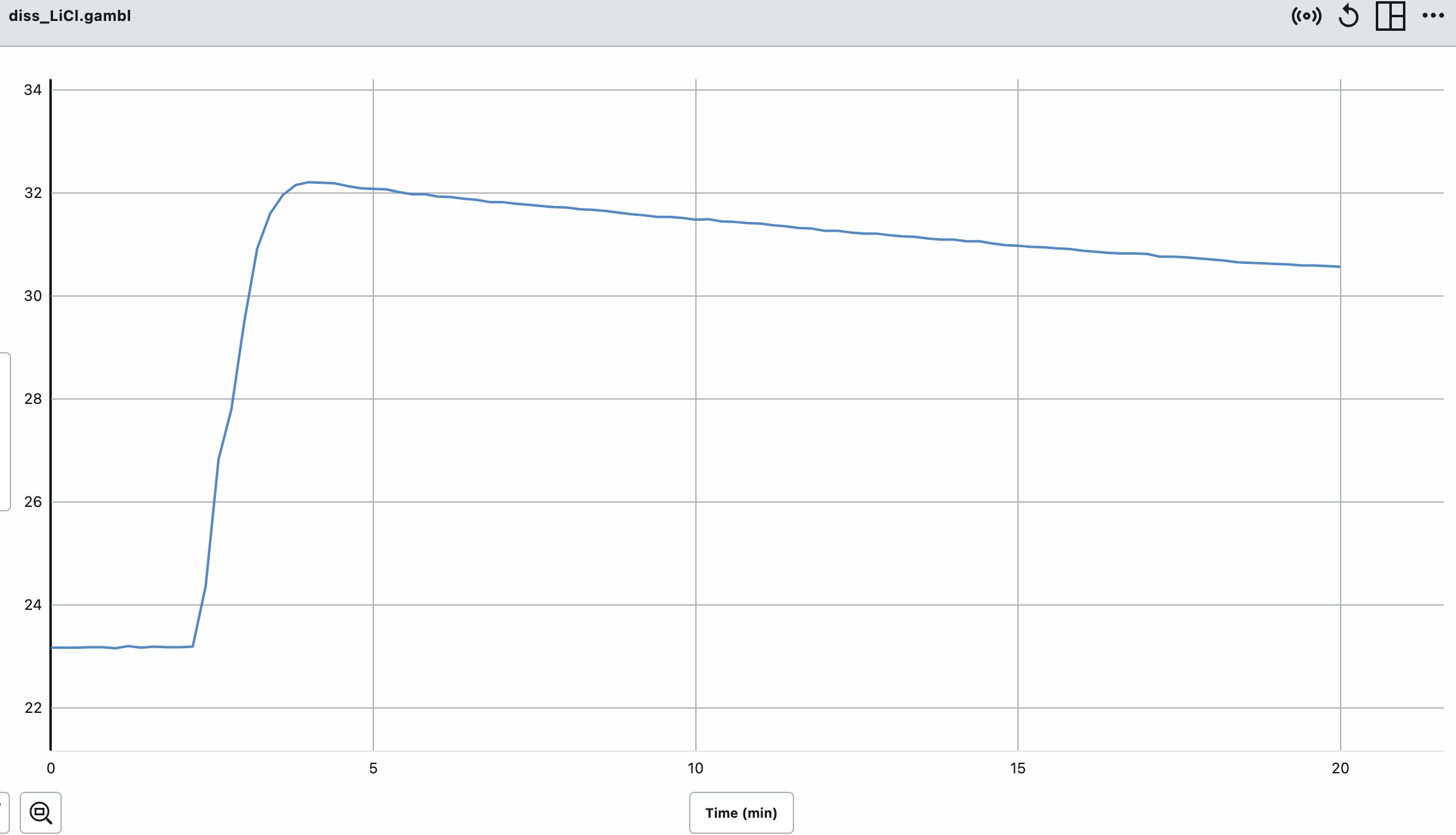 |
The following diagram illustrates the components of a calorimeter.
A chemistry lecture demonstration of dissolving calcium chloride, CaCl2, in water. Video courtesy of BerkeleyChemDemos. University of California Berkeley College of Chemistry Demonstrations Laboratory. B17 Pimentel Hall University of California Berkeley, CA 94720-1460
Please see the posted information about this demonstration on the CIDER web site.
If the enthalpy of hydration is greater than the enthalpy of the lattice, there is a net release of energy. The resultant solution gains this energy and the temperature of the solution increases.
We can calculate the thermal energy gained by the solution using q gained by the solution = m x c x ∆T
q is the thermal energy transferred, m is the total mass of solution, c is the specific heat capacity of the solution, and ∆T is the change in temperature of the solution.
This net release of energy is called the heat of solution (more properly the heat of dissolution) or the change in enthalpy of solution, ∆H solution. The change in enthalpy of solution for dissolving ionic salts is due to the net result of a series of steps: water molecules require an input of energy to separate, cations and anions in the lattice require an input of energy to separate, when an ion forms an interaction with a water molecule (ion-dipole interaction), energy is released.
We cannot directly measure the energy of hydrogen bonds breaking, electrostatic interactions breaking, interactions forming during the dissolving of an ionic salt. Bond breaking and bond forming or interactions breaking and forming has no mass. An application of the Law of Conservation of Energy allows one to infer the amount of energy released by the dissolving process based upon what energy was gained by the solution. The solution has total mass which can be measured, and a solution has an initial and final temperature which can be measured.
q released by the dissolving process + q gained by the solution = 0
q gained by the solution = -q released by the dissolving process
Students identify what is losing heat energy and what is gaining heat energy in this "experiment". For this experiment, the salt is poured directly into the water. This presents some difficulties in identifying the system and surrounding. There is no doubt that the resultant solution consists of the mass of water and the mass of the salt and combined this is the total mass of the solution. The entire solution increases in temperature. The water increases in temperature and the bulk chemical, CaCl2 (or LiCl), increases in temperature. We can calculate the thermal energy gained by the resultant solution. q solution = m c ∆T
where the q is the thermal energy transferred, m is the mass of the solution, c is the specific heat of the solution, and ∆T is the change in temperature of the solution.
For an exothermic reaction, the resultant solution gains thermal energy and the chemical reaction releases thermal energy.
The Law of Conservation of Energy is the central idea for any calorimetry experiment. We use an application of the Law of Conservation of Energy to model
qloss + qgain = 0 qgain = qsolution qloss = qrxn qsolution + qrxn = 0
qsolution = -qrxn
The change in enthalpy of the solution at constant pressure can be modeled by a simple equation
For a one-to-one stoichiometry chemical reaction system at constant pressure
∆Hrxn = qrxn /moles of limiting reagent (under conditions of constant pressure)
In a second calorimeter experiment we place solid ammonium chloride in water and stir. We notice the temperature of the solution decreases.
[information about ammonium chloride in water to be added]
I. Calorimetry Computer Simulation
The calorimeter computer simulation is designed to determine the heat of solution (or more properly the heat of dissolution) of various soluble solid ionic salts dissolved in water. The computer simulation has a particle view (molecular scene) animation of the ions, atoms and or molecules interacting during the dissolving process.
- Instructors and students can vary the mass of water and the mass of the solid.
- Record the temperature of the water and salt before adding the solid to the water.
- Record the temperature of the resultant solution after dissolving the solid.
This provides an opportunity for students to view a model of how energy is exchanged during a physical process. For example, when solid lithium chloride is placed in water initially the lithium cations and chloride anions in the LiCl lattice vibrate moderately and the water molecules move slowly. When the water molecules collide with the surface cations and anions the water molecules orient with the partial negative charge towards the lithium cations, Li+. An ion-dipole intermolecular force forms, releasing energy. An energy input separates a lithium, Li+, cation from a chloride, Cl-, anion. The water molecules with the newly formed ion-dipole IMF move faster. The temperature of the solution increases due to the faster movement of the water molecules (and associated ions) striking the thermometer. This process continues until all of the solid salt dissolves. This animation representing a dynamic particle interaction helps students to visualize energy transfer at the particle level. This is consistent with the recommendations of Alex Johnstone (1993) and other chemistry educators. Remember this visualization is model and it has limitations.
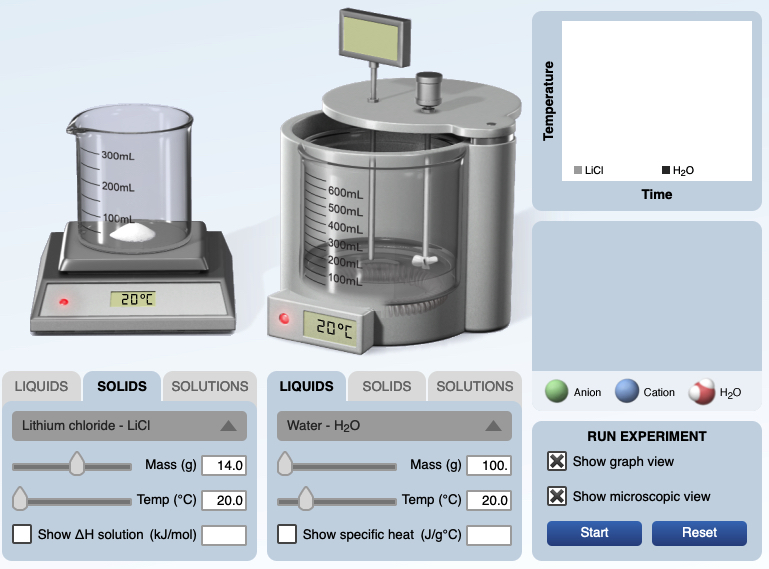 |  |
Currently, Firefox 111.0.1 is doing the best at playing the embedded animations (April 2023).
The HTML5 calorimetry computer simulation is available at the following URL. If you use the simulation, please cite the simulation. ©2016 Greenbowe, Abraham, Gelder Chemistry Education Instructional Resources. University of Oregon, Oklahoma State University, University of Oklahoma, Pearson.
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
J. Calculations of ∆H solution for dissolving lithium chloride in water using the data from the simulation of mixing LiCl(s) in water.
q solution = m c ∆T = 114.0 g x 4.184 J/g°C x 23.82°C = +11,351 J
q released by the dissolving process + q gained by the solution = 0
q gained by the solution = - q released by the dissolving process = -11,352 J
∆Hdissolving process = qdissolving process /moles of limiting reagent = -11,352 J/0.330 moles LiCl = -34.3 kJ/mol LiCl
∆H solution = ∆Hdissolving process
∆H solution = -34.3 kJ/mol LiCl
K. Calculations of ∆H solution for dissolving ammonium chloride (or nitrate) in water using the data from the simulation of mixing NH4Cl(s) in water or mixing NH4NO3(s) in water. The calorimeter simulation has ammonium nitrate.

For this experiment, the temperature of the resultant solution decreased. We infer from this that resultant solution released energy and the "dissolving process" absorbed energy.
∆T = T final - T initial = (10.26°C - 20.00°C) = -9.74°C
qsolution = m c ∆T = 126.0 g x 4.184 J/g°C x (-9.74°C) = -5,135 J
Application of the Law of Conservation of Energy
q absorbed by the dissolving process + q released by the solution = 0
q released by the solution = - q absorbed by the dissolving process
q absorbed by the dissolving process = -(-5,135 J) = + 5,135 J
for a one-to-one ratio of stoichiometry of the salt: NH4NO3 --> NH4+ + NO3-
Under conditions of constant pressure
∆H dissolving process = q dissolving process /moles of limiting reagent = +5,135 J / 0.200 moles NH4NO3
= +25,675 J/mol NH4NO3 = +25.6 kJ/mol NH4NO3
∆H solution = ∆H dissolving process
∆H solution = +25.6 kJ/mol NH4NO3
[add data for dissolving ammonium chloride in water here]
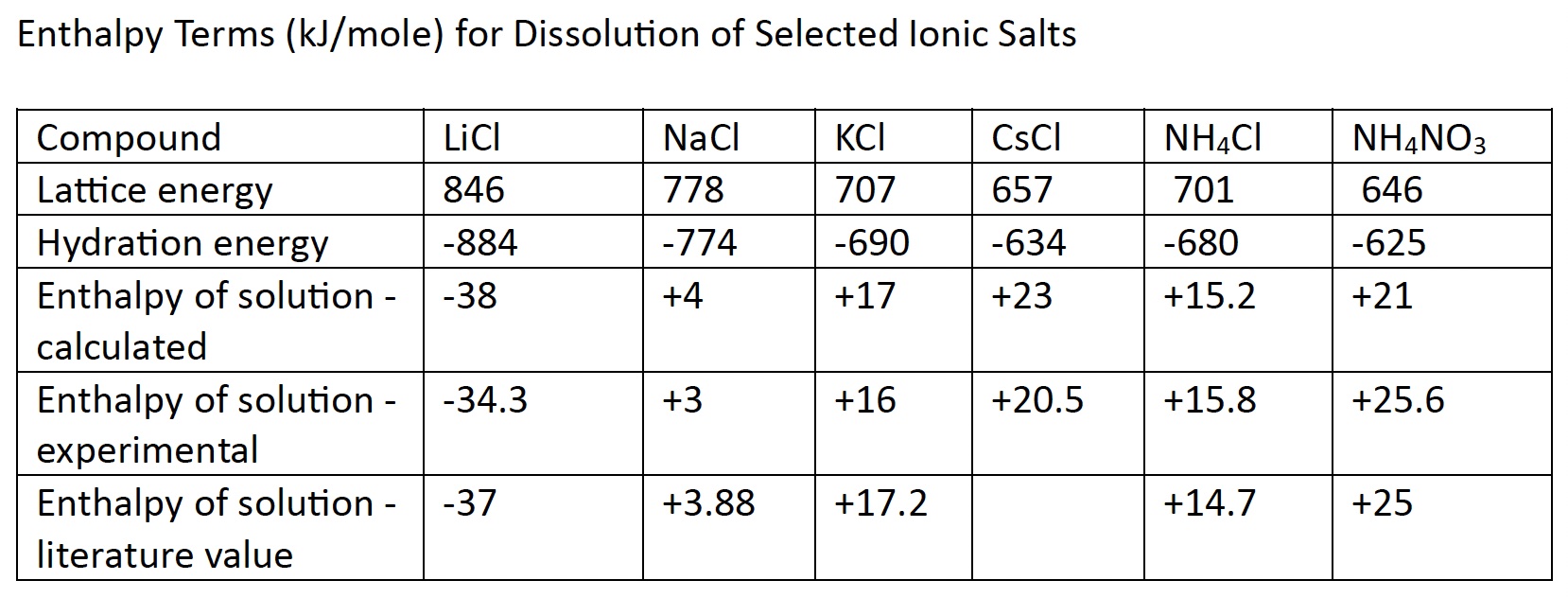
L. Lattice Energy
The following diagram on the left represents a view of a small portion of lithium chloride solid, LiCl(s). The small spheres are lithium ions, Li+, and the large spheres are chloride ions, Cl-. Opposite charges attract. The lithium cations and chloride anions are held together by strong electrostatic forces of attractions. It takes an input of energy to separate the positive ions from the negative ions. Coulomb's Law is applied to estimate the energy holding the ions together. The energy required to separate ions in a solid and take the ions into a gas phase is called lattice energy. During the dissolving process, ions are not in the gas phase. We will see how we still can make use of this information.
 | 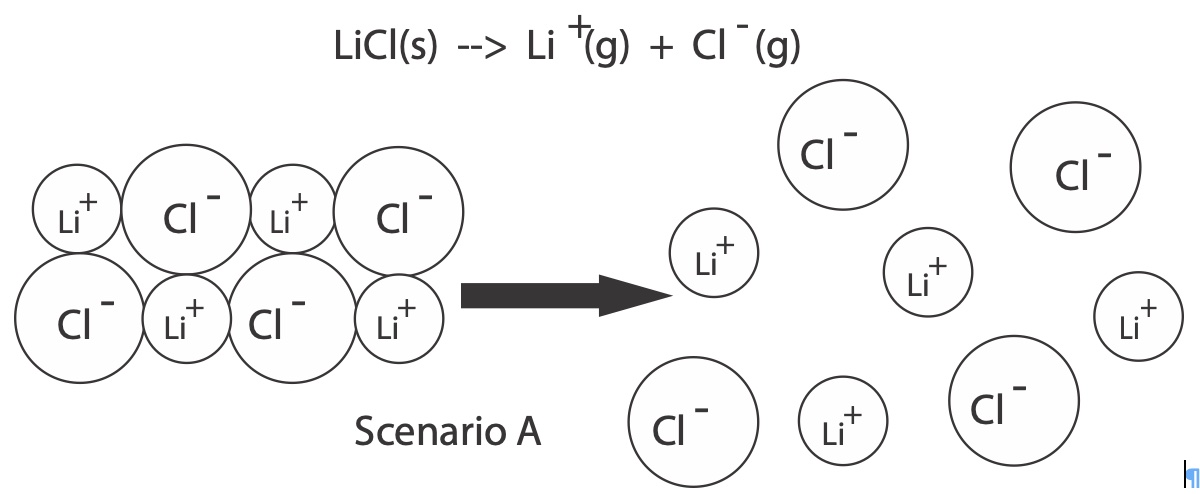 |
Lattice energy can be defined in two ways. One way is the enthalpy change that is required (input) to separate one mole of cations and anions in an ionic solid to create cations and anions in the gaseous state.
LiCl(s) --> Li+(g) + Cl-(g) ∆H lattice = + 37 kJ/mole

Another way to define lattice energy as the enthalpy change released (output) when one mole of cations and anions in the gaseous state come together to form one mole of an ionic solid.
Li+(g) + Cl-(g) --> LiCl(s) ∆H lattice = - 37 kJ/mole
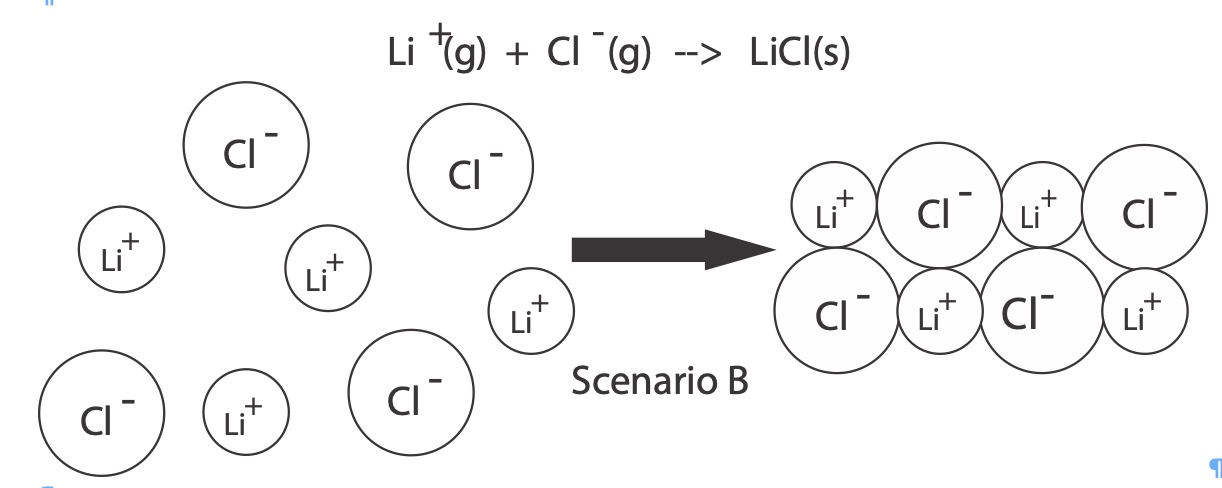
Each ionic salt has a unique lattice energy. Lattice energy is dependent on two main factors:
- the radius of the ions. The larger the ion size, the lower the lattice energy. For example, KCl(s) has lower lattice energy compared to NaCl(s). Coulomb's Force Law applies. The charge on the ions is either +1 or -1 in KCl and NaCl. However, the radius of the potassium ion is larger than the sodium ion. According to Coulomb's Force Law, the force of attraction decreases as the distance between charges increases.
- charge of ions. The greater the charge, the higher the lattice energy. For example, MgCl2 has greater lattice energy compared to NaCl. The radius of Mg2+ is about the same as Na+. According to Coulomb's Law because magnesium ion has a +2 charge, the chloride ion a -1 charge, the force of attraction will be about twice as much compared to the force of attraction between sodium ion which has a +1 charge and the chloride ion which has a -1 charge.
Table 15.1 Lattice Energies
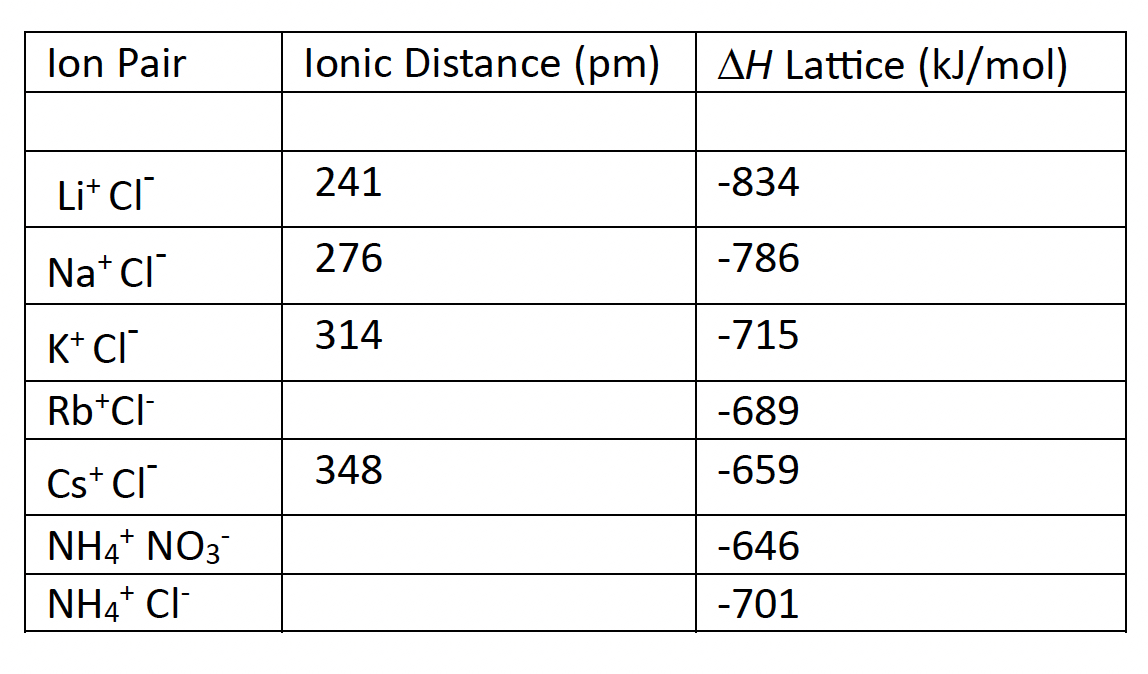
M. Heat of hydration
∆H hydration is the change in enthalpy released when one mole of an ion (cation or anion) forms ion-dipole IMFs with five, six or seven water molecules. The size of the ion plays a role in determining how many water molecules can surround the ion. The smaller the ion, the fewer water molecules can surround the ion.
 |  |
Each ion has a unique heat of hydration. Table 15.6 Heats of Hydration
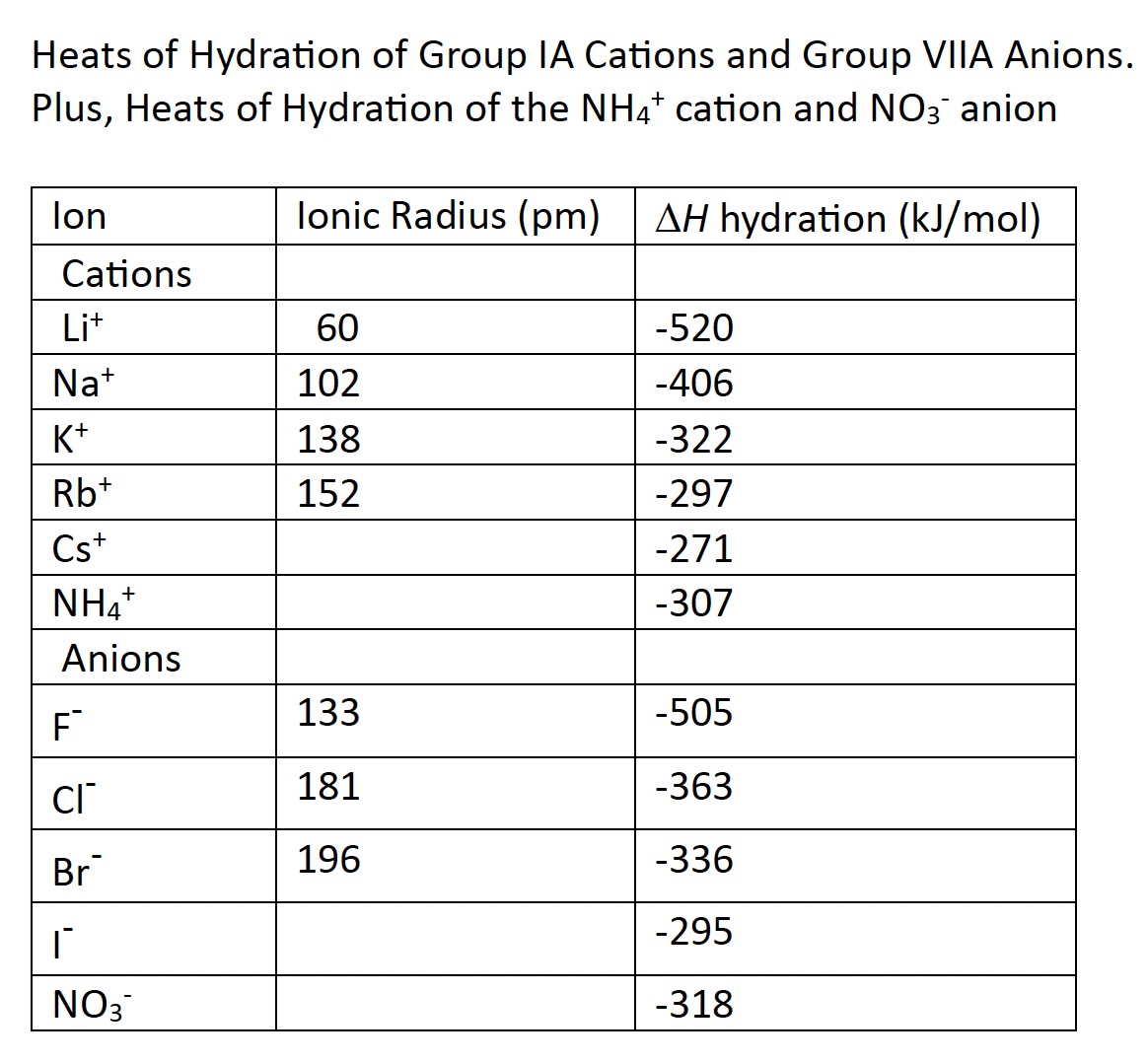
Show students the 3D Molecular Design Water molecules and the NaCl ionic model and how two types of ion-dipole IMFs form. As a reminder, the symbol Li+(aq) means a lithium cation is surrounded by five or six water molecules in aqueous solution. The lithium cation is small. The oxygen end of a water molecule is directed at the positive lithium ion. This is called hydration. The symbol Cl-(aq) means a chloride anion is surrounded by six or seven water molecules in aqueous solution. The water molecules are oriented in a specific way. The partial positive charged hydrogen atoms in a water molecule are oriented toward the negatively charged chloride ions.

N. Enthalpy Diagrams
The dissolution process causes a change in enthalpy of the solution, ∆H solution. The change in enthalpy is defined as the enthalpy before mixing minus the enthalpy after mixing
∆H solution = H before mixing - H after dissolution
We cannot measure individual enthalpy values, H, but we can determine the change in enthalpy, ∆H .
In Tro, Chemistry AMA 5th edition Solutions, different color spheres are used to represent solute and solvent particles. In this model there are three separate energy steps.
Separation of solute particles requires an input of energy to break the solute-solute bonds. An endothermic process.
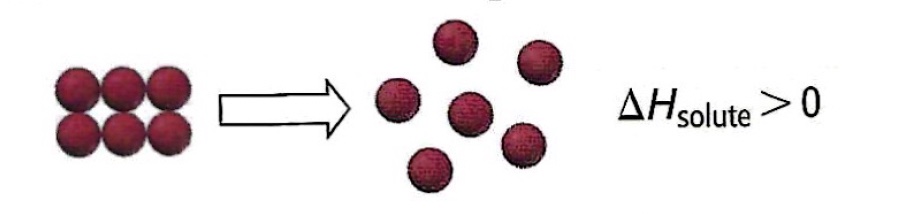
Separation of solvent particles requires an input of energy to break the solvent-solvent bonds. An endothermic process.
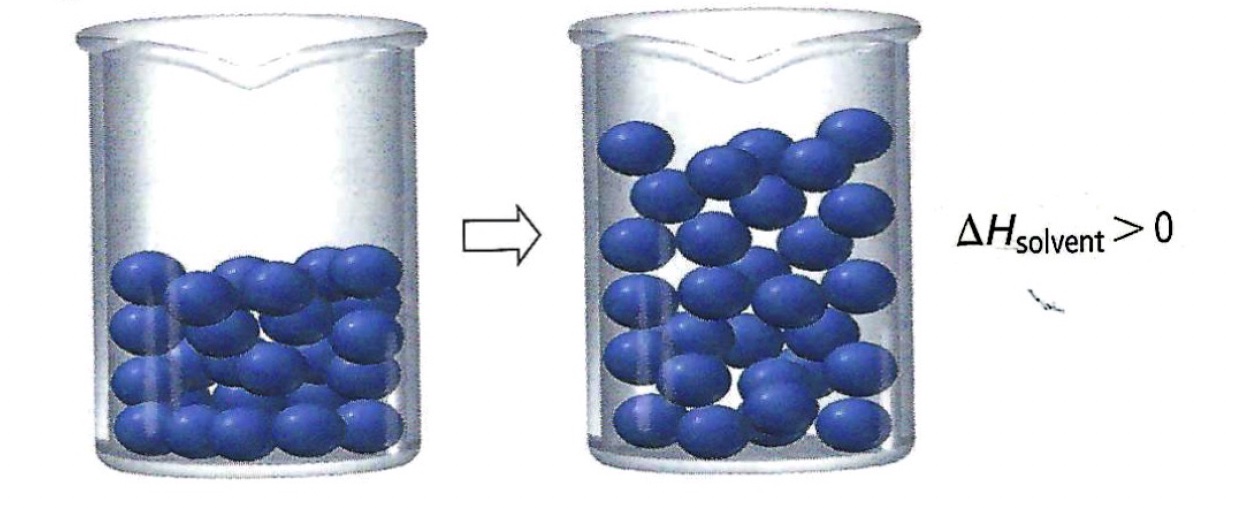
Mixing solute and solvent particles releases energy. An exothermic process.
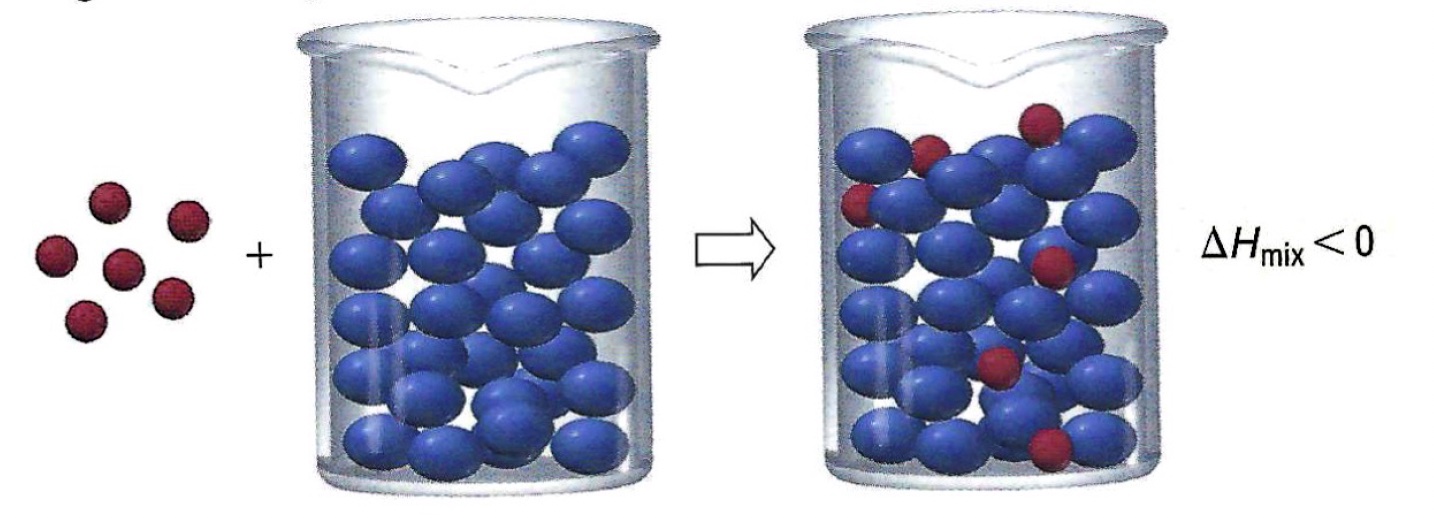
Diagram, Tro, N. (2020). Chemistry: A Molecular Approach, 6th edition; Pearson, Hoboken, NJ.
There are two types of enthalpy diagrams used to represent dissolving a salt in water. The first type is a continuation of this theoretical model. Enthalpy of solute (the lattice energy of the salt) and enthalpy of solvent (water) as well as the enthalpy of the mixture (heat of hydration) are included in this first type of enthalpy diagrams.
Along the vertical axis (y-axis), lattice energy is represented qualitatively by an upward arrow. The enthalpy diagrams indicate the endothermic steps, enthalpy of solute (which is the lattice energy and enthalpy of the solvent (separation of water molecules). And an exothermic step, mixing the separated ions and separated water molecules to form a solution.
If the mixing step releases more energy compared to the endothermic steps, the overall dissolving process is exothermic.
∆H solution = ∆H solute + ∆H solvent + ∆H mix
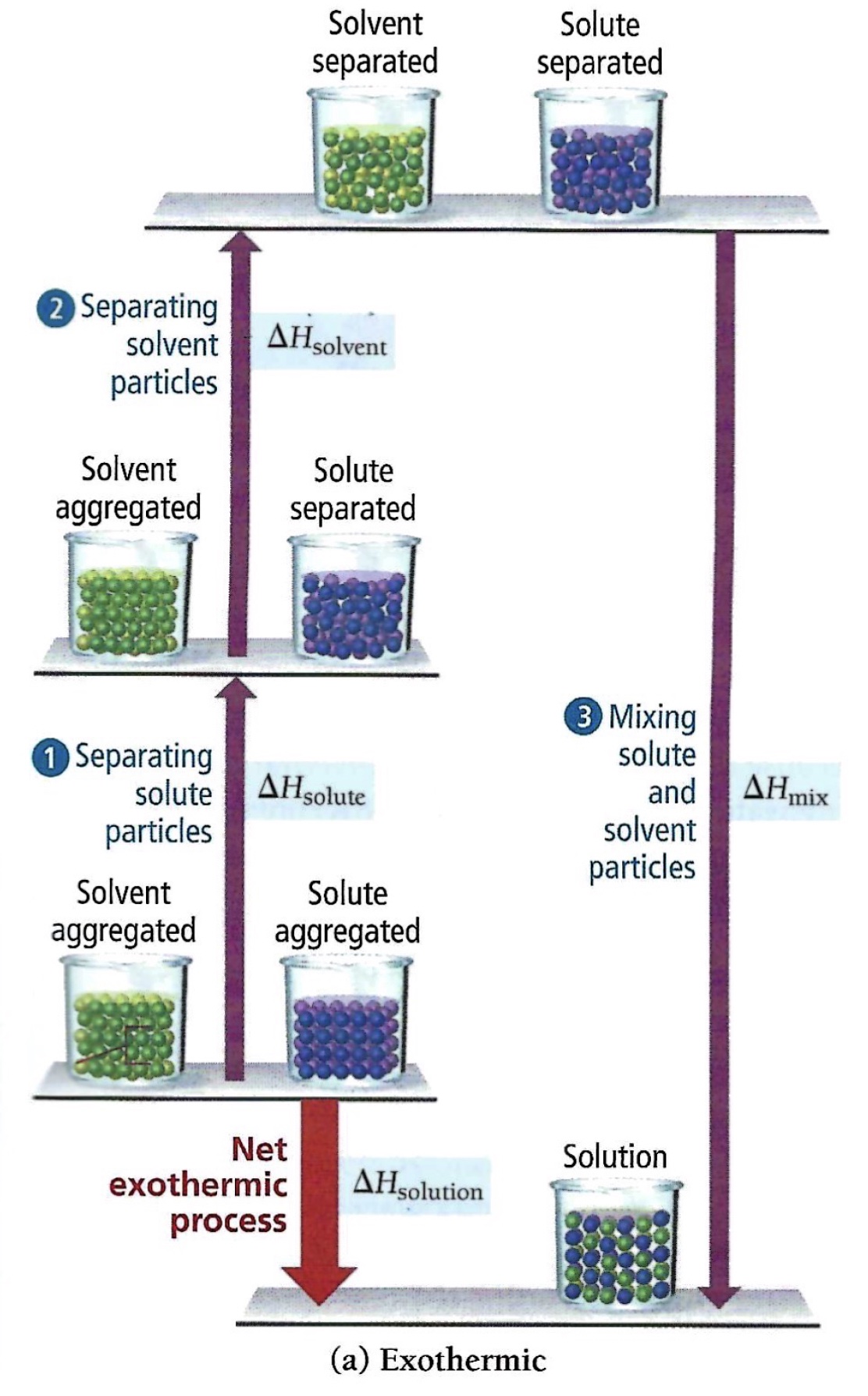
Diagram, Tro, N. (2020). Chemistry: A Molecular Approach, 6th edition; Pearson, Hoboken, NJ.
If the mixing step releases less energy compared to the endothermic steps, the overall dissolving process is endothermic.
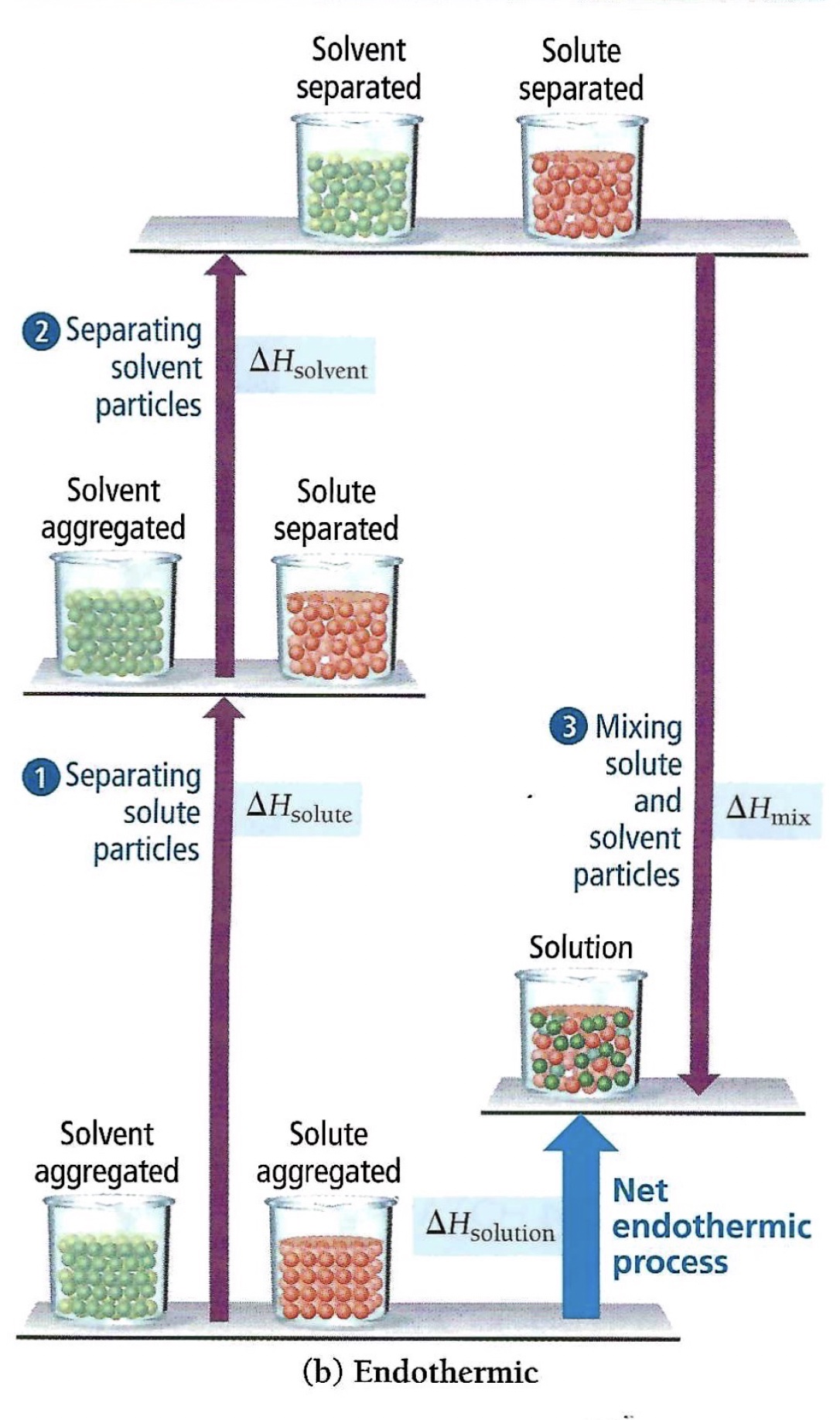
Diagram, Tro, N. (2020). Chemistry: A Molecular Approach, 6th edition; Pearson, Hoboken, NJ.
∆H solution = ∆H solute + ∆H solvent + ∆H mix
Enthalpy Diagram Representing the Energy Involved in the Dissolving Process for LiCl: A Theoretical Model
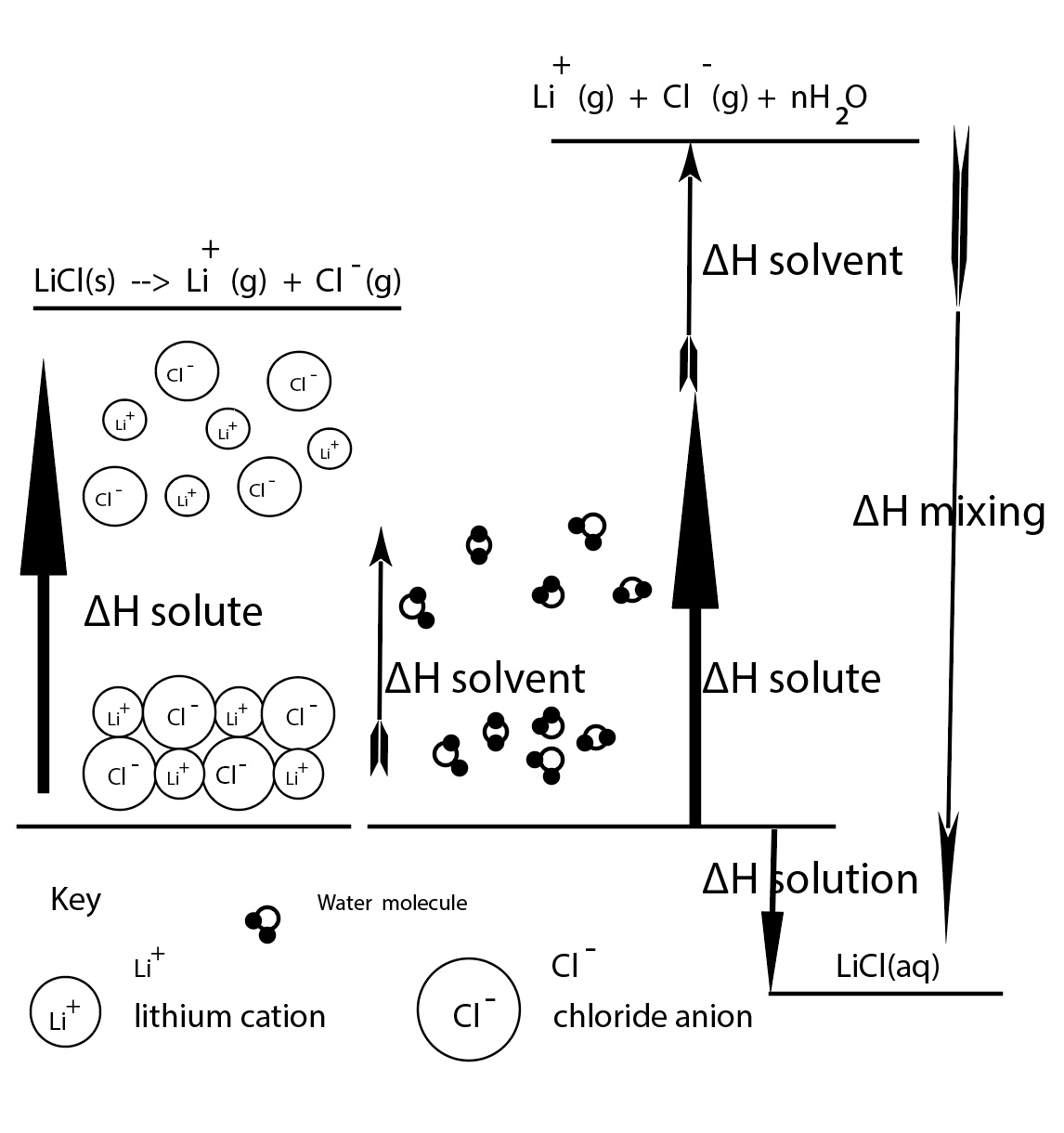
Dissolving a salt in water does not force the cations and anions into the gas phase. It is difficult to measure ∆H solvent + ∆H mix individually.
In practice, a slight adjustment is made. We can combine ∆H solvent and ∆H mix and this will equal ∆H hydration.
∆H hydration = ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H hydration or ∆H solution = ∆H solute + ∆H hydration
O. Enthalpy Diagram Representing the Energy Involved in the Dissolving Process for LiCl and NH4Cl: A model
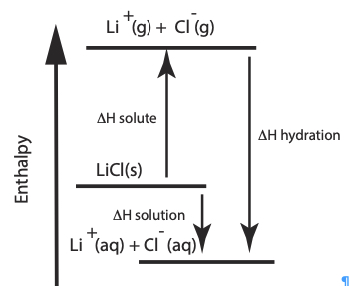 | 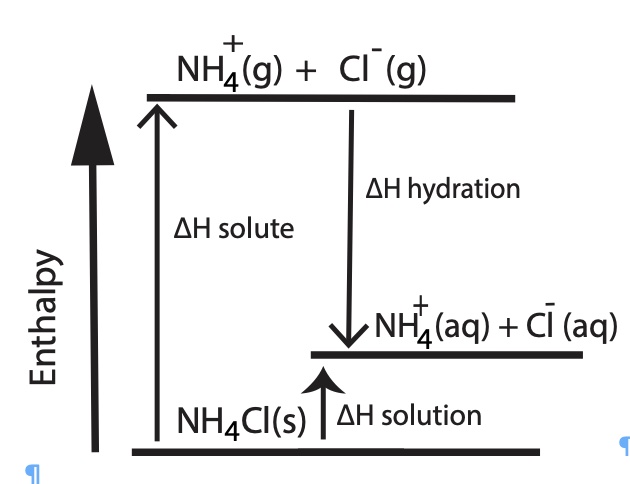 |
The above two enthalpy diagrams help students visualize how it is possible for a salt to have either an overall endothermic dissolving process, change in enthalpy of solution, ∆Hsolution or an overall exothermic process. Lithium chloride has an overall exothermic dissolving process. The heat of hydration of LiCl is greater than the lattice energy, this dissolving process is exothermic. Ammonium chloride has an overall endothermic dissolving process. The heat of hydration is less than the heat of solute (lattice energy), the dissolving process, the heat of solution, is endothermic. One driving force for dissolving a salt is the formation of aqueous species. One other driving force is an increase in entropy.
Calculation of ∆H solution for dissolving lithium chloride in water using ∆H lattice and ∆H hydration values.
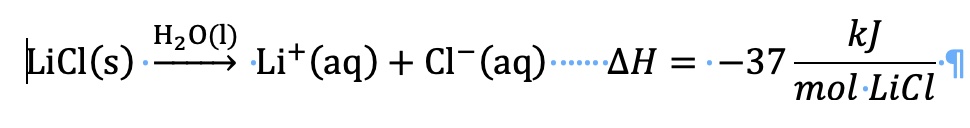
The lattice energy is defined as the energy required to separate one mole of LiCl(s) into gas phase ions. For this calculation, the lattice energy is a positive value. An input of energy is required to separate cations from anions.
∆H solution = ∆H lattice + ∆H hydration
∆H solution = +853 kJ/mol + (-883 kJ/mol) = -30. kJ/mol LiCl
Compare the heat of solution using two different methods.
Using values for ∆H lattice and ∆H hydration, ∆H solution = -30. kJ/mol LiCl
Using measurements and calculation from a calorimeter experiment
∆H dissolving process = ∆H solution = -34.3 kJ/mol LiCl
The literature value for ∆H solution for dissolving LiCl in water is -37.03 kJ/mol LiCl
P. Calculation of ∆H solution for dissolving ammonium chloride in water using ∆H lattice and ∆H hydration values.
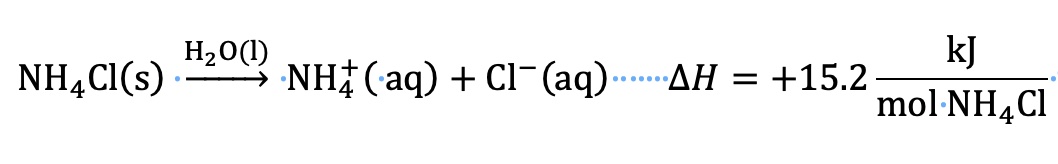
The lattice energy is defined as the energy required to separate one mole of NH4Cl (s) into gas phase ions. For this calculation, the lattice energy is a positive value. An input of energy is required to separate cations from anions.
∆H solution = ∆H lattice + ∆H hydration
∆H solution = +705 kJ/mol + (-689.8 kJ/mol) = +15.2. kJ/mol NH4Cl
Compare the heat of solution using two different methods.
Using values for ∆H lattice and ∆H hydration, ∆H solution = +15.2. kJ/mol NH4Cl
Using measurements and calculation from a calorimeter experiment
∆H dissolving process = ∆H solution = +15.8 kJ/mol NH4Cl
The literature value for ∆H solution for dissolving NH4Cl in water is +14.77 kJ/mol NH4Cl
Q. Summary
With respect to the various energy inputs and outputs - here are the steps for dissolving a salt in water:
- It takes an input of energy to separate water molecules. ∆H solvent
- It takes an input of energy to separate cations from anions in the solid structure and to take the ions into the gas phase. (lattice energy, ∆H lattice)
- Energy is released when ion-dipole IMFs form between ions and water molecules. The solvent and solute particles mix. (∆H mix )
- In theory, the change in enthalpy of solution can be determined
∆H solution = ∆H lattice + ∆H solvent + ∆H mix
- We can combine ∆H solvent and ∆H mix and this will equal ∆H hydration. ∆H hydration = ∆H solvent + ∆H mix
∆H solution = ∆H lattice + ∆H hydration
- When the heat of hydration of a salt is greater than the heat of solute (lattice energy), the dissolving process is exothermic.
- When the heat of hydration of a salt is less than the heat of solute (lattice energy), the dissolving process is endothermic.
- There is either a net output of energy or a net absorption of energy for the overall dissolving process - this is the change in enthalpy of the solution (heat of solution).
- In this model, we are not taking into account the change in entropy.
Assessment
[information to be added]
R. Prerequisite Knowledge
[information to be added]
S. Learning Objectives
1. Use experimental data to develop a conceptual understanding of one aspect of Law of Conservation of Energy and how to apply it to calorimeter experiments:
q lost + q gain = 0
2. Identify whether a dissolving process is exothermic or endothermic.
3. When an ionic salt dissolves in water, explain how energy is transferred at the particle level.
4. Use enthalpy diagrams to represent dissolving processes. Determine ∆H solution from an enthalpy diagram.
5. Identify what gains thermal energy and what loses thermal energy in a calorimetry experiment.
6. For a physical process, such as dissolving a salt in water, explain how energy is transferred (released or absorbed) at the molecular level.
7. In a calorimetry experiment conducted at constant pressure, given the total mass of the solution, specific heat of the solution, and change in temperature of the solution, calculate the thermal energy gained or released by a solution, q solution = m c ∆T
8. When the calorimetry experiment is carried out under constant pressure conditions, calculate ∆H solution for the dissolving process.
9. Given the change in enthalpy for a reaction, the amounts of reactants, and a balanced chemical equation, calculate the heat exchanged for a reaction.
10. Draw a representation of a hydrated cation and a hydrated anion using six water molecules. Be sure to show the correct orientation.
T. AP Chem Learning Objectives
Learning objective 5.4 The student is able to use conservation of energy to relate the magnitudes of the energy changes occurring in two or more interacting systems, including identification of the systems, the type (heat versus work), or the direction of energy flow.
Learning objective 5.5 The student is able to use conservation of energy to relate the magnitudes of the energy changes when two nonreacting substances are mixed or brought into contact with one another.
Learning objective 5.6 The student is able to use calculations or estimations to relate energy changes associated with heating/cooling a substance to the heat capacity.
U. Making this presentation interactive - active learning
The instructor should "frame" the instructional activity accompanying this computer simulation. There are several in-class POGIL-like activity to accompany this computer simulation. A recommendation is to use several POGIL activities - calorimetry, lattice energy, coulomb's law, etc. {specify}
A teacher guide and a student activity sheet are available for this lesson.
There are a set of interactive guided-inquiry Power Point slides to accompany this computer simulation. There are clicker questions that can accompany this computer simulation.
Student have difficulties with several concepts associated with thermochemistry (see reference #1 below).
Students do not have difficulty with the idea that the amount of reactants influences the thermal eneregy exchanged, but they do have difficulty applying this concept.
There are several calorimetry lecture demonstrations that can be used to accompany this computer simulation.
V. References
Abell, Timothy N.; Bretz, Stacey Lowery (2019). Development of the Enthalpy and Entropy in Dissolution and Precipitation Inventory. Journal of Chemical Education, v96 n9 p1804-1812.
Bain, Kinsey; Towns, Marcy H. (2018).Investigation of Undergraduate and Graduate Chemistry Students' Understanding of Thermodynamic Driving Forces in Chemical Reactions and Dissolution. Journal of Chemical Education, v95 (4), p512-520.
Brown, LeMay, Bursten, et al. Chemistry: The Central Science 13th ed. 2015. Pearson: Hoboken, NJ.
Greenbowe, T.J. and Meltzer, D.E. (2003). “Student learning of thermochemical concepts in the context of solution calorimetry.” International Journal of Science Education, 25(7), 779-800.
Johnstone, A. H. (1991). Why is science difficult to learn? things are seldom what they seem. Journal of Computer Assisted Learning, 7(2), 75–83. https://doi.org/10.1111/j.1365-2729.
Johnstone, A. H. (1982). Macro- and micro-chemistry. School Science Review, 64, 377–379.
Knight, R. (2008). Physics for Scientists and Engineers: A Strategic Approach, 2nd edition. Pearson Addison-Wesley: San Francisco, CA.
Malkoc, Ummuhan. (2017). Investigating Teachers' Understanding of the Salt Dissolution Process: A Multi-Media Approach in Education. Turkish Online Journal of Educational Technology - TOJET, v16 n1 p55-71.
Naah, B. M., & Sanger, M. J. (2012). Investigating students’ understanding of the dissolving process. Journal of Science Education and Technology, 22(2), 103–112.
Russell, JW, Kozma, RB, Jones, J Wykoff, N Marx, J Davis (1997). Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts Journal of Chemical Education, 74 (3), 330.
N. Tro (2023) Chemistry: A Molecular Approach (6th ed), Pearson: Hoboken, NJ.
Zumdahl, S., Zumdahl, S. (2014) Chemistry (9th edition, AP edition), Brooks-Cole Cengage: Belmont, CA
*********
©2010 Greenbowe Chemistry Education Instructional Resources, University of Oregon, Department of Chemistry & Biochemistry, Eugene, Oregon 97403
