Web page author: T. Greenbowe, University of Oregon. This page is under construction.
Summary II A copper-copper electrolytic cell is built comprised of two copper electrodes in 1.0 M CuSO4(aq) connected to a DC voltage-current generator. The initial mass of both electrodes are measured. Students observe two shiny copper metal electrodes at the start of the demonstration. An ammeter measures the applied current. When current is applied to the electrolysis cell copper(II) ions are reduced to copper atoms at the cathode and copper atoms are oxidized to copper(II) ions at the anode. Within two minutes students observe discoloration on both of the copper electrodes. The time to plate copper metal on the cathode is recorded along with the current (amps). At the end of the electroysis experiment, the mass of both electrodes are measured. Faraday's Law of electrolysis is applied. Students calculate the moles of electrons passed in the cell, the moles of copper plated on the cathode, and the mass of copper plated. The calculated theoretical mass of copper produced on the cathode is compared to the actual mass of copper plated on the cathode.
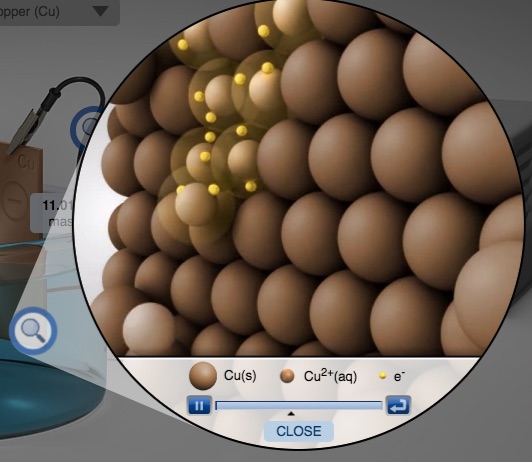
A companion computer simulation is also a component of the demonstration. In particular, there are particle animations representing the oxidation half-reaction that occurs at the anode and the reduction half-reaction that occurs at the cathode. A hand-out is available - students start with a blank electrolysis cell and identify the components of an electrolysis cell. These components afford an opportunity for students to make connections among the macroscopic, microscopic (particle), and symbolic levels of representation (A. Johnstone, 1993).
 |  |
 |  |
This demonstration when accompanied with the Electrolysis Class Activity and Electrolysis Computer Simulation serves well as an introduction to the basic principles of electrolysis.
Students' conceptual and quantitative understanding of electrolysis improves when an active learning approach involving exploring a simple copper-copper electrolysis cell. The simple half-reactions involved in this copper-copper cell provides a good starting place for students to learn about the concepts associated with electrolysis cells. Having students work through the questions and problems on the activity sheet, interacting with the electrolysis computer simulation and animations, and doing clicker questions throughout the presentation or lesson has proven to be more effective compared to didactic lecturing about electrolysis.
Time Estimates Please allow 12-15 minutes for the electrolysis cell to run. The instructor and students can be working on the calculations and diagram the electrolysis cell while the cell is operating. Allow 20-22 minutes if you are integrating the accompanying guided-inquiry activity, computer simulation, animations, and the demonstration.
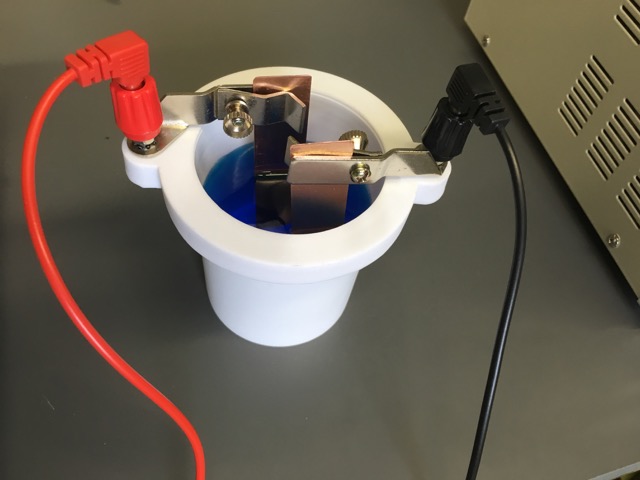
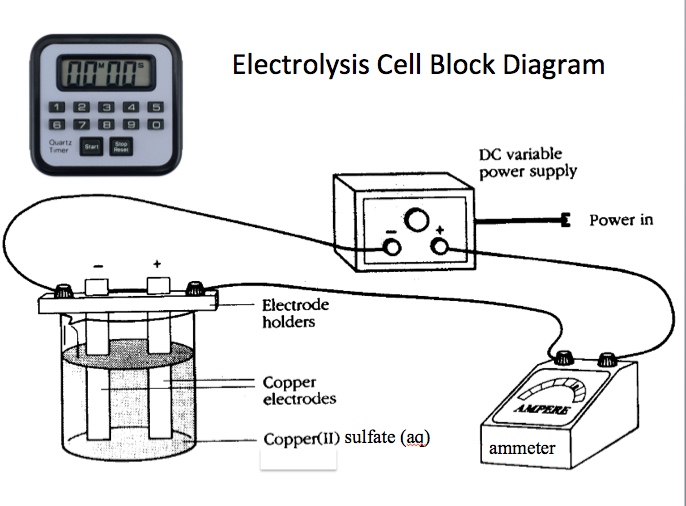
Description of the Demonstration Mentioning refining copper metal and showing a photograph or image of refining copper metal by electrolysis helps to set the context for this activity. Most students do not see any logical reason why one would want to plate copper metal onto a copper electrode. A postage balance is used to measure the mass of two copper electrodes prior to the demonstration. The electrodes are placed in 1.0 M CuSO4(aq) solution. The top of one Cu electrode is connected to the negative terminal of a D.C. Power Supply. The other Cu electrode is connected to the positive terminal of the D.C. Power Supply.
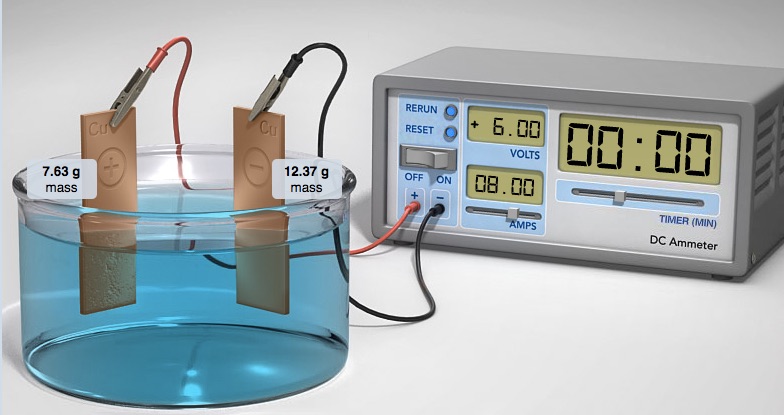
When current is applied to the electrolysis cell copper(II) ions in solution are reduced to copper atoms at the cathode. Copper atoms on the anode are oxidized to copper(II) ions. The cathode gains mass, the anode looses mass. When the experiment ends, the electrodes are dried and the mass of each electrode weighed on the mini-balance. The time to plate the metal and the current (amps) applied is used to calculate the theoretical amount of mass that should plate on the cathode. The actual mass obtained is compared to the theoretical amount calculated. The mass of copper gained at the cathode should be equal to the mass of copper lost at the anode.
Preparation Notes
Materials: two clean copper electrodes, 60 mL 1.0 M CuSO4(aq), electrolysis cell, red and black wire leads with alligator clips, ammeter, stop watch, mini electronic balance to measure the mass of the electrodes, AC power strip, hair dryer, paper towels, steel wool, scouring powder, laboratory brush, 100 mL of deionized water, Q-tips, and a DC Power supply with ability to change current and set a constant current.


Examples of DC Power supplies with ability to change the current and to set a constant current.
Procedure for the Demonstrator
A. Prepare the electrodes. Prepare the electrodes at least 3 hours before the demonstration. Use steel wool to clean the copper metal electrodes. The cleanliness of the metal determines the uniformity of the electroplating. Clean the electrodes with scouring powder (i.e. Comet or Ajax) and a laboratory brush. Handle the electrodes by the edges to avoid finger prints on the electrode surface or use laboratory gloves. Rinse the electrodes with deionized water. Allow the electrodes to dry.
The electrodes are placed in 1.0 M CuSO4(aq) solution. The top of one Cu electrode is connected to the negative terminal of a D.C. Power Supply. The other Cu electrode is connected to the positive terminal of the D.C. Power Supply. When current is applied to the electrolysis cell copper(II) ions in solution are reduced to copper atoms at the cathode. Copper atoms on the anode are oxidized to copper(II) ions. The cathode gains mass, the anode looses mass. When the experiment ends, the electrodes are dried and the mass of each electrode weighed on the mini-balance. The time to plate the metal and the the current (amps) applied is used to calculate the theoretical amount of mass that should plate on the cathode. The actual mass obtained is compared to the theoretical amount calculated.
Safety Notes. Copper(II) sulfate solutions are corrosive. Solid copper sulfate causes skin and eye irritation. Wash immediately after accidental contact. Using standard safe laboratory practices, dilute solutions of copper (II) sulfate are not considered a significant hazard.
Clean Up: Return the copper(II) sulfate solution (electrolyte) to the stock bottle for reuse. Use a Q-tip to make sure that the alligator clips are clean and dry.
During the operation of the electrolysis cell, students would work to calculate the theoretical mass of copper plated on the cathode. Students also can predict the moles of electrons passed, the moles of copper plated, and the mass of copper plated when the current is applied for half of the time.
The effectiveness of this Electrolysis Cell demonstration can be enhanced when it is accompanied by an electrolysis cell computer simulation and computer animation at the particulate level (atom level). One idea instructors should emphasize is the balance of charge at all times in the copper(II) sulfate solution.
Definitions
The process of electrolysis involves forcing a current (electrons) through an electrochemical cell to cause a thermodynamically unfavorable oxidation-reduction reaction to occur. Electrical work generated by a direct current power supply (or a battery) can cause a thermodynamically unfavorable oxidation-reduction chemical reaction to occur.
Direct Current Power Supply is an electrical device where electrons are pulled into the terminal marked positive "+" and forced out of the terminal marked negative, "-".
Current is defined as the charge passing a point per unit of time. The unit of current is ampere. For electrolysis experiments we are interested in the number of electrons that pass a specific point in time.
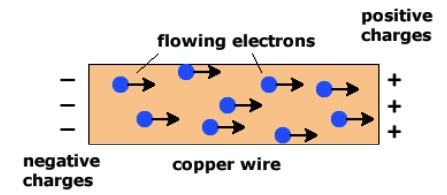
When a battery is connecred to a circuit, the battery does work and pushes electrons through a wire. In a circuit, electrons move are pushed out from the negative terminal of a battery and pulled in towards the positive terminal of a battery.
Current x time = total electrical charge in units of amp-seconds. 1 amp-sec = 1 Coulomb of charge
I x t = Q
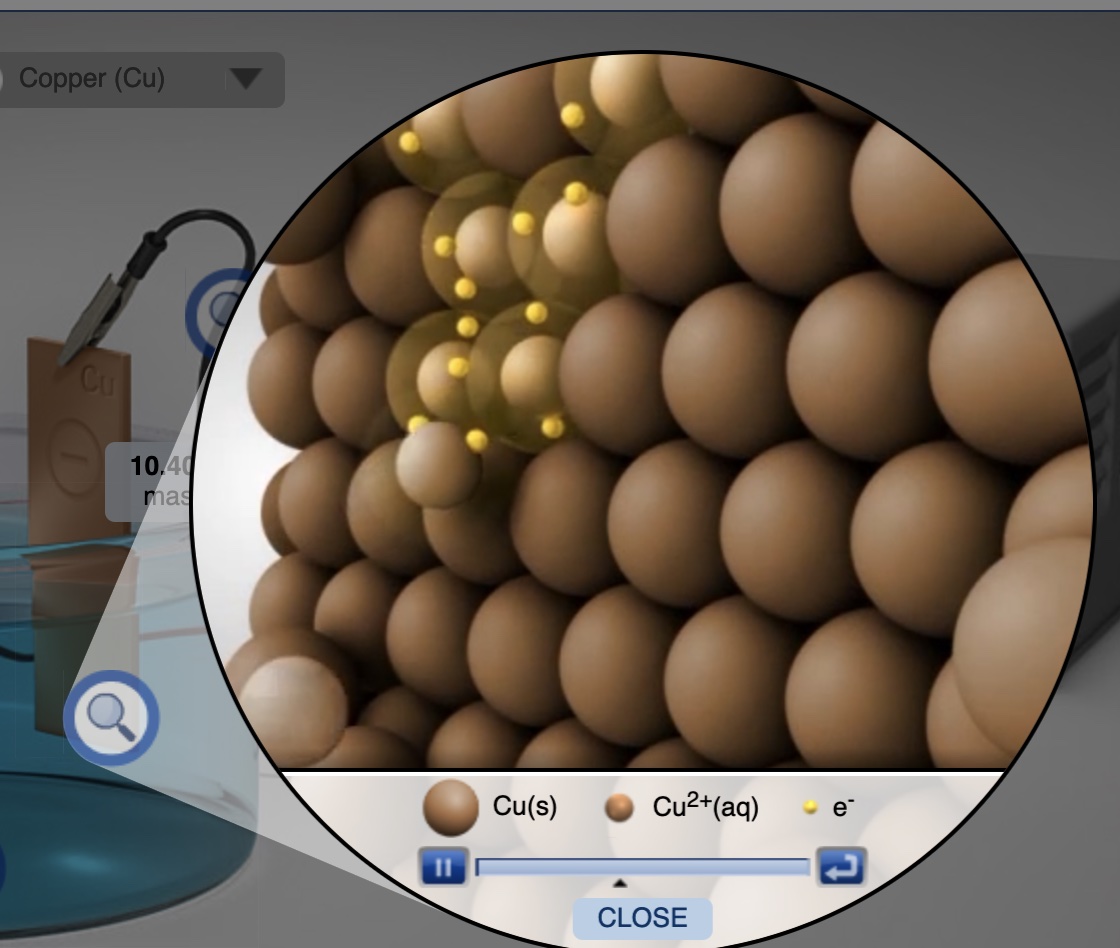
Plating means using electrolysis to deposit neutral metal atoms on an electrode (cathode) by reducing metal ions in solution. In the following example of a copper-copper electrolysis cell, when operating copper metal is plated on the copper electrode attached to the black terminal (ground terminal) of the DC Power supply. Electrons are forced out of the terminal marked negative, "-", into the circuit and into the electrode.
Representing a copper-copper electrolysis cell
 |  |
Reduction of Cu2+ ions to Cu atoms at the cathode.
Student diagram of a copper-copper electrolysis cell
Chemical Concepts
What is the relationship between electrical quantities and the amount of substance electrolyzed?
There are three variables involved in electrolysis of plating metals: time, current, and type of ion/metal. Data may be collected prior to the demonstration or quickly generated using the electrolysis simulation.
What is the relationship between mass plated and the amount of current applied and while keeping time constant? Vary the current while keeping time constant and measure the mass of copper plated. Plot a graph of mass of copper versus current.
What is the relationship between mass plated and the time current was applied while keeping the current at a constant value? Vary the time while keeping current constant and measure the mass of the copper plated. Plot a graph of mass of copper versus time.
What is the relationship between type of metal ion in solution and moles of metal plated given the same current and time? Vary the type of metal ion in solution (Al3+, Cu2+, Ag+) while keeping current constant and measure the mass of the metal plated. Calculate the moles of metal. Plot a graph of moles of metal versus number of electrons involved per atom.
Student Activity Sheet #XY. During the operation of the electrolysis cell, given the research questions and data sets, students can work to calculate the theoretical mass of copper plated on the cathode. Students also can predict the moles of electrons passed, the moles of copper plated, and the mass of copper plated when the current is applied for various timef adn currents.
What is the relationship between mass plated and current applied when time and type of metal ion are held constant? time = 12.0 minutes, Cu2+(aq)
| Time (min) | Current (A) | Mass Cu (g) | Mole Cu |
| 12.0 | 1.00 | 0.241 | 0.00379 |
| 12.0 | 2.00 | 0.476 | 0.00749 |
| 12.0 | 3.00 | 0.721 | 0.0113 |
| 12.0 | 4.00 | 0.958 | 0.0151 |
Identify the variables
Independent = the current of the cell Dependent = moles of copper plated (or collected)
Controlled = the time the cell is run (the total amount of time the current was applied)
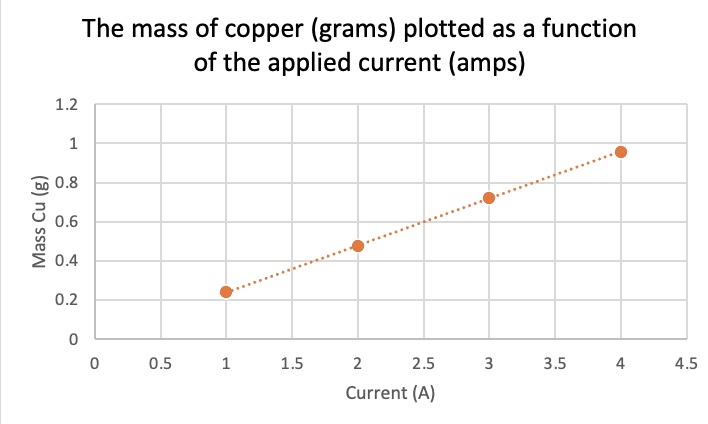
The relationship between mass and current is a direct linear proportion. The slope of the line is a constant.
mass ∝ current mass = Z x current y = mx + b
What is the relationship bewtween mass plated and time allocated when current and type of metal ion are held constant? Current = 2.00 amps, ion = Cu2+(aq)
| Current (A) | Time (min) | Mass Cu (grams) | Moles Cu |
| 2.00 | 1.00 | 0.04 | |
| 2.00 | 2.00 | 0.08 | |
| 2.00 | 3.00 | 0.12 | |
| 2.00 | 4.00 | 0.16 | |
| 2.00 | 6.00 | 0.24 |
Identify the variables
Independent = the time the cell is run (the total amount of time the current was applied)
Dependent = moles of copper plated (or collected)
Controlled = the current of the cell
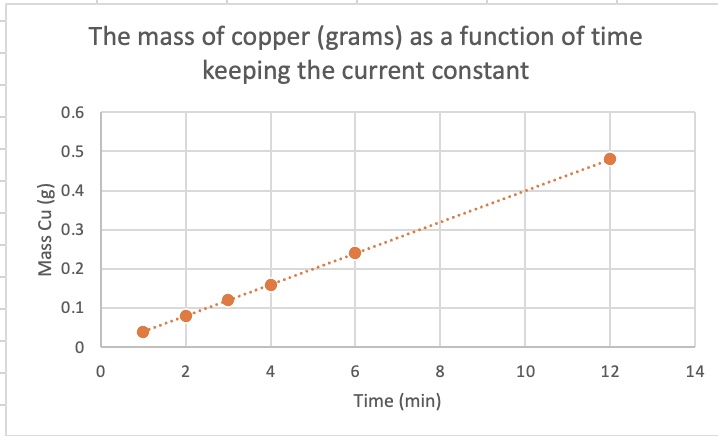
The relationship between mass and time is a direct linear proportion. The slope of the line is a constant.
mass ∝ time mass = k x time y = mx + b
ssssss Compare four different metals - same current, same time = same # of moles of electrons
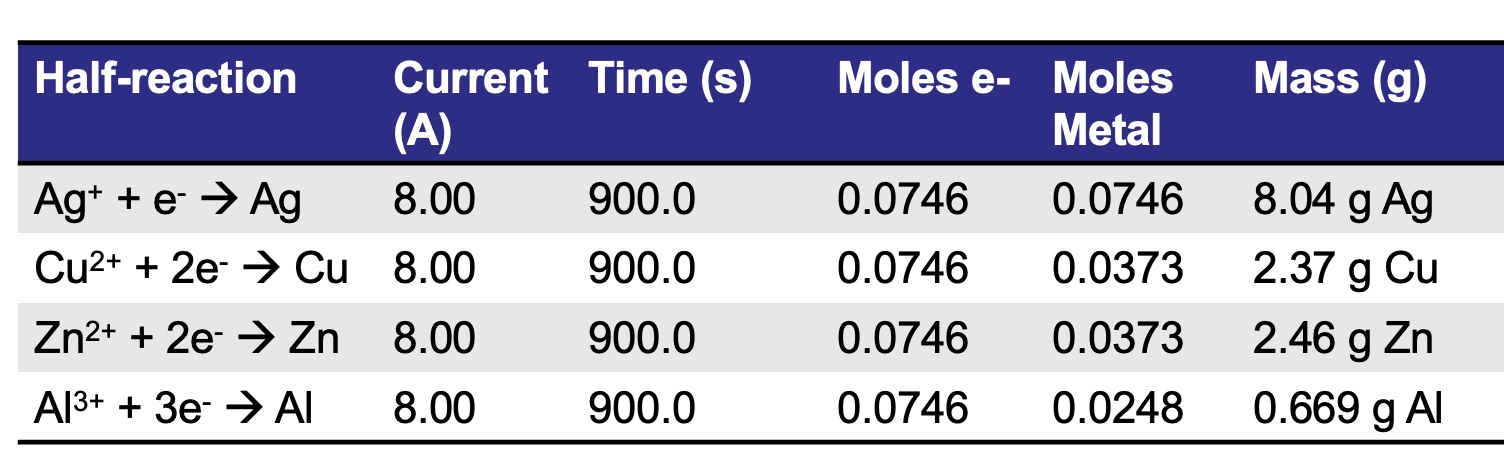
There are three times the moles of metal plated for silver compared to aluminum when the same number of electrons are transferred.
When the charge of an ion is larger compared to another ion, i.e. Al3+ vs Ag+, it takes more electrons to reduce the ion to an atom. The same charge results in fewer atoms being formed.
2 Al3+ + 6 e- -> 2 Al when six electrons are passed through an electrolysis cell, two aluminum atoms are produced
3 Cu2+ + 6 e- -> 3 Cu when six electrons are passed through an electrolysis cell, three copper atoms are produced
6 Ag+ + 6 e- -> 6 Ag when six electrons are passed through an electrolysis cell, six silver atoms are produced
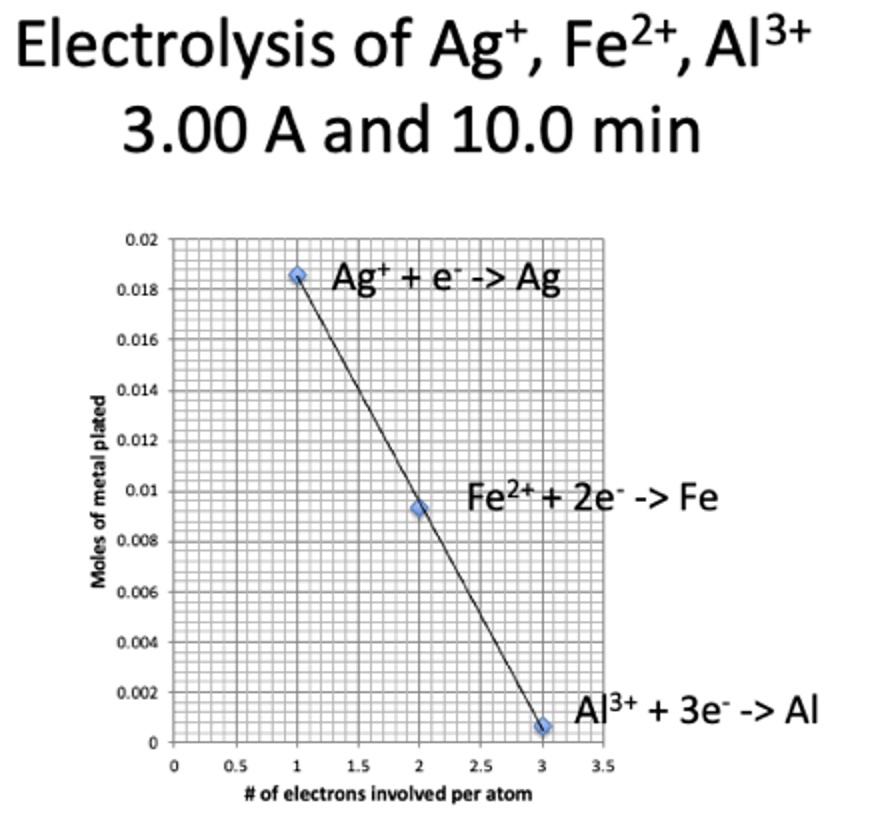
There is an inverse linear relationship between ion charge and moles of electrons.
moles of metal plated ∝ 1/# of electrons involved per atom moles metal = Z' x 1/# of electrons involved per atom y = mx + b
Analysis of the data
mass of metal plated is directly proportional to the amount of current applied at a constant time. there is a linear relationship between mass and current.
mass of metal plated is directly proportional to the time a constant current is applied. there is a linear relationship between mass and time.
mass of metal plated is inversely proportional to the number of electrons transferred per atom at constant current and time.
A monovalent ion requires 1 electron for discharge, a divalent ion requires 2 electrons for discharge a trivalent ion requires 3 electrons for discharge. Thus, if x electrons flow, atoms are discharged.
Cu2+ + 2e- -> Cu when two electrons are passed through an electrolysis cell, one copper atom is produced
3 Cu2+ + 6 e- -> 3 Cu when six electrons are passed through an electrolysis cell, three copper atoms are produced
Cu2+ + 2e- -> Cu when two moles of electrons are passed through an electrolysis cell, one mole of copper atoms are produced
Total charge versus moles of electrons
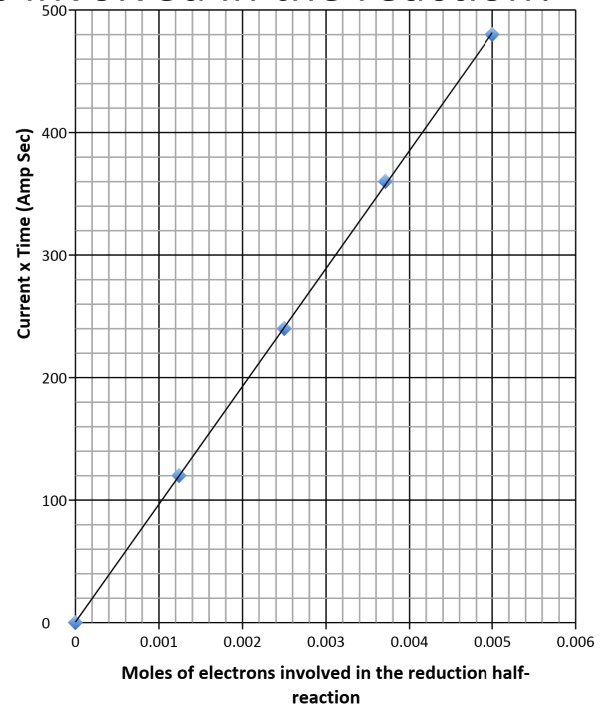
The slope of the line, m, in the graph above has a linear relationship, y = mx +b, where b = 0
current x time ∝ moles of electrons applied
y = mx +b
current x time = m x moles of electrons applied + b
slope = m = current x time / moles of electrons applied during electrolysis
m = ∆y/∆x calculate the slope of the line
m = [200.0 amp-sec - 100.0 amp-sec]/ [0.00206 moles e - 0.00102 moles e] = 96,153 amp-sec/mole e- = 96,153 Coulombs/mole e-
The Faraday constant, F, is 96,485 Coulombs/mole e-
The mass of metal, m, electrolyzed is
mass ∝ time mass = k x time
mass ∝ current mass = Z x current
mass ∝ current x time (Q, total charge)
mass = c x Q
moles of metal = mass x (1/Molar Mass) = mass/M
moles of metal x M = mass of metal
moles of metal x M = constant x Q
where
- NA is the Avogadro constant;
- Q = xe is the total charge, equal to the number of electrons (x) times the elementary charge e;
- F is the Faraday constant.
Faraday's Laws of Electrolysis
1. The quantity of a chemical substance electrolyzed is proportional to the amount of charge (number of coulombs) applied. The number of electrons passed through an electrolysis cell determines the amount of substance electrolyzed.
The product of constant current multiplied by the time of passing (total charge, Q) is proportional to the mass, m, electrolyzed (Langford and Beebe, 1969).
mass ∝ Q mass/Q = Z
The constant of proportionality, Z, is called the electrochemical equivalent (ECE) of the substance. The ECE can be defined as the mass of the substance deposited/liberated per unit charge.
2. The masses of different elements electrolyzed when a given amount of charge is applied are in the ratio of the equivalent weights of those elements.
mass ∝ E E = molar mass/valence
the mass of the substance liberated/deposited at the electrodes is directly proportional to their chemical equivalent/
E is the molar mass (M) divided by the valence (v).
The Significance of Faraday's Electrolysis Discoveries
Faraday's Law(s) of Electrolysis are of great significance because the scientific data supported the claim for the existance of atoms (supporting Dalton's Atomic Theory) and the existance of a fundamental units of matter - the atom and the electron. At the time of his experimental work, Faraday was skeptical of the atomic theory.
Faraday was aware that the results of his electrolysis experiment showed that a "fixed quantity of electricity liberated one equivalent weight (a mole of monovalent substance) of any species implied a strong connection between electricity and the atomic theory" (Langford and Beebe, 1999).
Faraday (1839) "The equivalent weights of bodies are simply those qaunetities of them which contain equal quantities of electricity. Or, if we adopt the atomic theory . . . the atoms of bodies which are equivalent to each other in ordinary chemical reactions, have equal quantities of electricity associated with them."
There is a microscopic unit of charge associated with the valence of atoms.
G. Johnstone Stoney (1874) "the reason that a fundamental unit of charge is associated with the valence unit of an atom in electrolysis is that the charge itself comes in 'atoms'. Stoney named this electrical atom the electron" (Langford and Beebe, 1999).
Calculation of Mass Electrolyzed (Deposited) Road Map
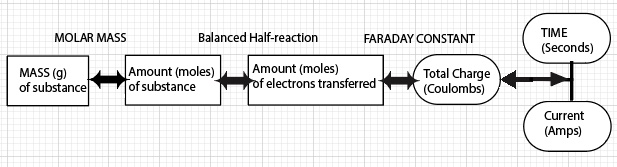
Data for a copper-copper electrolysis cell
| Time (min) | Current (A) | Mass Cu (g) | Mole Cu |
| 12.0 | 1.00 | 0.241 | 0.00379 |
| 12.0 | 2.00 | 0.476 | 0.00749 |
How many moles of electrons are passed through a copper-copper electrolysis cell when the cell operated for 12.0 minutes at a constant current of 2.00 amps?
12.0 minutes x 60.0 seconds/minute = 720. seconds I x t = Q 1 amp-sec = 1 Coulomb of charge
Current x time = 2.00 amps x 720. seconds = 1,440 amp seconds x 1 Coulomb/ 1 amp sec = 1,440 Coulombs
1,440 Coulombs x 1 mole of e-/ 96,485 Coulombs = 0.0149 mole of e-
Calculate the mass of copper deposited on the cathode of a copper-copper electrolysis cell when operated under the conditions above.
Cu2+ + 2e- -> Cu when two moles of electrons are passed through an electrolysis cell, one mole of copper atoms are produced
0.0149 mole of e- x 1 mole Cu/2 mole e- = 0.00745 mole Cu
0.00745 mole Cu x 63.54 g/mole Cu = 0.473 grams Cu
Chemical Concepts Working backwards
Student Activity Sheet #XX. During the operation of the electrolysis cell, students can work to calculate the theoretical mass of copper plated on the cathode. Students also can predict the moles of electrons passed, the moles of copper plated, and the mass of copper plated when the current is applied for half of the time.
Table 17.7 Sample Data and Results the Mass of Copper Metal Produced by an Electrolysis Cell
Oxidation half-reaction: Cu -> Cu2+ + 2e- Reduction Half-reaction: Cu2+ + 2e- -> Cu
Current (A) | Time (s) | Moles e- | Moles Metal | Mass of Metal (g) | |
8.00 | 900.0 | 0.0746 | 0.0373 | ||
8.00 | 450.0 | 0.0373 | 0.0186 |
Table 17.7 Sample Data and Results of Calculations of a Copper-Copper Electrolysis Cell
| Reduction Half-reaction | Current (A) | Time (s) | Moles e- | Moles Metal | Mass of Metal (g) |
| Cu2+ + 2e- -> Cu | 8.00 | 900.0 | 0.0746 | 0.0746 | |
| Cu2+ + 2e- -> Cu | 8.00 | 450.0 | 0.0373 | 0.0373 | |
Learning Objectives
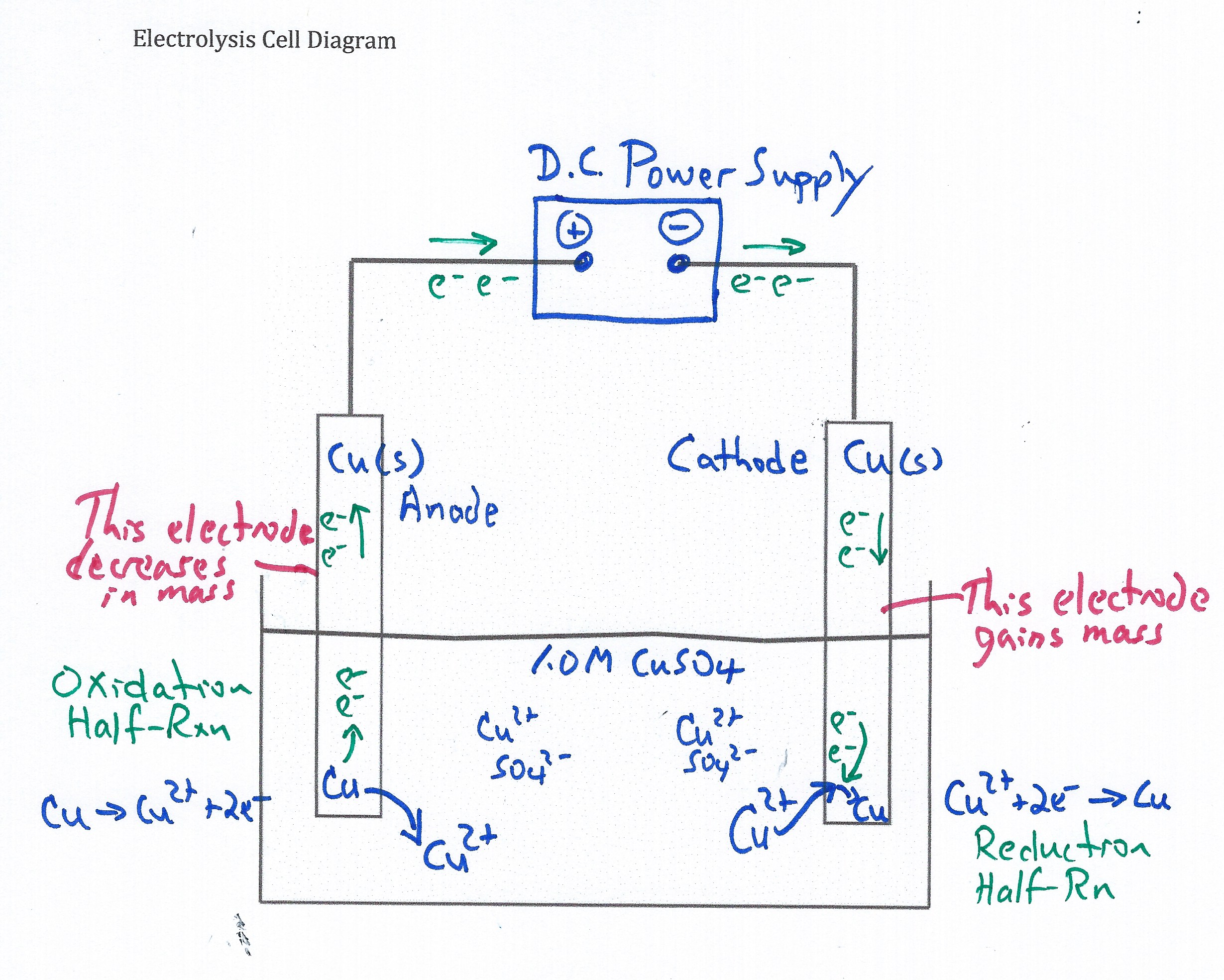
1. Given a diagram of an electrolytic cell, identify the anode, cathode, direction of which electrons and ions move, the location of the oxidation half-reaction, the location of the reduction half reaction.
2. Given a description or a diagram of an electrolytic cell, write the oxidation half-reaction and the reduction half-reaction.
3. Relate the amount of product(s) generated in an electrolytic cell to the stoichiometry of the reduction half-reaction and to the amount of electrical charge passed in the cell.
4. At the particle level of representation (atom level), show how the number of electrons involved in a single reduction half-reaction, i.e. Cu2+ + 2e- -> Cu, scales up to the mole level: i.e. 1 mole Cu2+ + 2 mole e- -> one mole Cu.
5. Distinguish the terms "current", "voltage", "electromotive force (emf"), "potential difference", and "standard reduction potential". Current is the number of electrons transferred or passed in a cell. Voltage is the force or the striking ability of the electrons.
6. The migration of ions in the electrolyte solution of an electrolysis cell is the movement of charge, which is equivalent to the movement of elections in a wire in a circuit.
7. Calculate the mass of product produced during electrolysis given the stoichiometry, the amount of electrical current passed in a specific time in the cell.
8. Calculate the quantity of of charged passed in an electrolytic cell, given the stoichiometry, and the amount of electrical current passed in a specific time in the cell.
9. Determine the relationship among coulombs, faradays, time, and reduction-half reaction for an electrolysis cell.
Curriculum Notes
With respect to the active learning Class Activity, provide students with the "Electrolysis Model", physical constants, and an empty diagram of an electrolysis cell. Have students complete the electrolysis cell diagram as the instructor sets-up the demonstration. Have students identify the flow of electrons into and out of the DC Power supply, the direction of ion migration in the cell, half-reactions occurring at each electrode, the cathode, the anode, and which electrode gains mass.
This demonstration, when paired with the electrolysis computer simulation, provides a great opportunity for students to experience the three levels of representation: microscopic, macroscopic, and symbolic (Johnstone's Triangle).
Student Difficulties (Misconceptions) with Electrolysis. The first sentence is the misconception or student difficulty. The second sentence is a statement of the correct science.
1. When identical metal electrodes are used in an electrolysis cell, the same reactions occurs at both electrodes and the products are the same at both electrodes.
When identical metal electrodes are used in an electrolysis cell, the electrode connected to the positive terminal of a D.C. (direct current) power supply or battery serves as the anode (site of oxidation Cu --> Cu2+ + 2e-) and the electrode connected to the negative terminal of a D.C. (direct current) power supply serves as the cathode (site of reduction: Cu2+ + 2e- __> Cu ).
2. The power source used in an electrolytic cell pulls electrons in at the negative terminal and pushes electrons out at the positive terminal. When a direct current power source is used in an electrolysis cell, electrons are forced out of the negative terminal and pulled in at the positive terminal.
3. During electrolysis, electrons move through electrolyte solution by being attracted to positive ions in the solution. During electrolysis, there is a mass migration of cations and anions in opposite direction in the electrolytic solution. The cations migrate toward the cathode and the anions migrate toward the anode. Electrons do not travel from one electrode to the other through an aqueous electrolyte solution.
4. During electrolysis, no reaction will occur if two inert electrodes are used. While the material of an inert electrode does not react, an inert electrode serves as a conduit for the electrons to interact with species in the electrolyte solution.
5. In electrolytic cells, oxidation occurs at the cathode and reduction occurs at the anode. In all electrochemical cells, oxidation occurs at the anode and reduction occurs at the cathode.
6. In electrolytic cells, water is unreactive and does not undergo oxidation and reduction. In electrolytic cells, of there are species present that are more reactive compared to water. Water can undergo an oxidation-reduction reaction - the electrolysis of water will generate hydrogen gas and oxygen gas.
7. When writing the chemical reaction that occurs in an electrolytic cell, the half-cell reactions are reversed prior to combining them. When writing the chemical reaction that occurs in an electrolytic cell, the half-cell reactions are combined.
8. The calculated cell potentials in electrolytic cells can be positive. Since the reactions involved in electrolysis cells are always "non-spontaneous", the calculated cell potentials in electrolytic cells are negative.
9. In electrolytic cells, the direction of the applied voltage has no effect on the reaction or the site of the anode and cathode. In electrolytic cells, the direction of the applied voltage determines the site of the anode and cathode.
10. The number of electrons transferred in an electrolysis cell is proportional to the emf or volts, i.e. more electrons transferred means a higher voltage. The number of electrons transferred in an electrolysis cell is proportional to the current (amps) and time, i.e. the higher the amperage for a longer time means more electrons transferred.
The number of electrons transferred in an electrochemical cell is the charge in units of Coulombs. The current is the number of electrons that passes through an electrochemical cell in a given period of time. 1 Coulomb per second is 1 ampere. 1 amp = 1 Coulomb/sec
References
De Jong, O.D. and Treagust, D. (2002). "The teaching and learning of electrochemistry." Chapter 14 pages 317- 337. In Chemical Education: Toward Research-based Practice Eds. Gilbert, J.K., De Jong, O., Justi, R., Treagust, D., Van Driel, J.H. Kluwer Academic Publishers, Boston.
Garnett, P. J., & Treagust, D. F. (1992). "Conceptual difficulties experienced by senior high school students of electrochemistry: Electrochemical (galvanic) and electrolytic cells." Journal of Research in Science Teaching, 29(10), 1079-1099.
Gelder, J.I., Abraham, M.R., Greenbowe, T.J. (2015). “Teaching electrolysis with guided-inquiry.” In Sputnik to Smartphones: A Half-Century of Chemistry Education, M. Orna (ed.) ACS Symposium Series, Volume 1208, pp 141-154. American Chemical Society: Washington, D.C.
Johnstone, A. H. 1993. The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9): 701-705.
Loh A. S. L., Subramaniam R. and Tan K. C. D., (2014), Exploring students’ understanding of electrochemical cells using an enhanced two-tier diagnostic instrument, Res. Sci.Technol. Educ., 32(3), 229–250.
Ozkaya, A.R., Uce, M., Sahin, M. (2003). Prospective teachers’ conceptual understanding of electrochemistry: galvanic and electrolytic cells. University Chemistry Education, 7, 1-12.
Penelope Ann Huddle, Margaret Dawn White, and Fiona Rogers, “Using a Teaching Model to Correct Known Misconceptions in Electrochemistry,” J. Chem. Educ., Vol. 77, 2000, 104−110.
Sia, D., Treagust, D., Chandrasegaran, A. (2012). “High school students’ proficiency and confidence levels in displaying their understanding of basic electrolytic concepts." International Journal of Science And Mathematics Education, Dec, Vol.10(6), pp.1325-1345.
Sanger, M.J. and Greenbowe, T.J. (2000). “Addressing Student Misconceptions Concerning Electron Flow in Electrolyte Solutions with Instruction Including Computer Animations and Conceptual Change Strategies.” International Journal of Science Education, 22, 521-537.
Sanger, M.J. and Greenbowe, T.J. (1997). “Student Misconceptions in Electrochemistry: Current Flow in Electrolyte Solutions and the Salt Bridge.” Journal of Chemical Education, 74(7), 819-823.
Sanger, M. J. and Greenbowe, T.J. (1997). “Common Student Misconceptions in Electrochemistry: Galvanic, Electrolytic, and Concentration Cells.” Journal of Research in Science Teaching, 34(4), 377-398.
Strong, F.C. (1961). Faraday's Laws in One Equation. Journal of Chemical Education. 38 (2): 98.


