Electrolysis Computer Simulation HTML5
Electrolysis computer simulation of a various metal-metal electrolytic cells. Choose metal electrodes, electrolyte, current and time. The simulation shows the change of mass for the anode and cathode. For example, set-up a zinc electrode and copper electrode in aqueous zinc nitrate. Set the current at 8 amps and the time at 15 minutes. After 15 minutes (simulated time), the copper electrode gained 2.44 grams in mass and the zinc electrode lost 2.44 grams of mass. Zinc metal in observed to plate on the copper electrode.


http://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/html5/Electro/Electro.php
Greenbowe, T., Abraham, M., Gelder J. (2016). Electrolysis Computer Simulation, Pearson: Hoboken, NJ.
If you use the simulation, cite the simulation. The simulation may not be used in any lesson sold for profit.
Available metals: aluminum, zinc, iron, copper, silver
Available aqueous solutions: aluminum nitrate, zinc nitrate, iron(II) nitrate, copper(II) nitrate, silver nitrate
Examples of set-ups: zinc-copper electrodes in zinc nitrate solution, plate zinc on copper
copper-copper electrodes in copper(II) nitrate solution, plate copper on copper
There is a student activity sheet that accompanies this computer simulation.
This versatile computer simulation can be used as part of a lecture presentation, POGIL classroom activity, as a component of a laboratory experiment involving electrolysis and electrochemistry, as an enhancement of lecture demonstrations, as a make-up laboratory experiment, as part of an end-of-chapter homework assignment, etc.
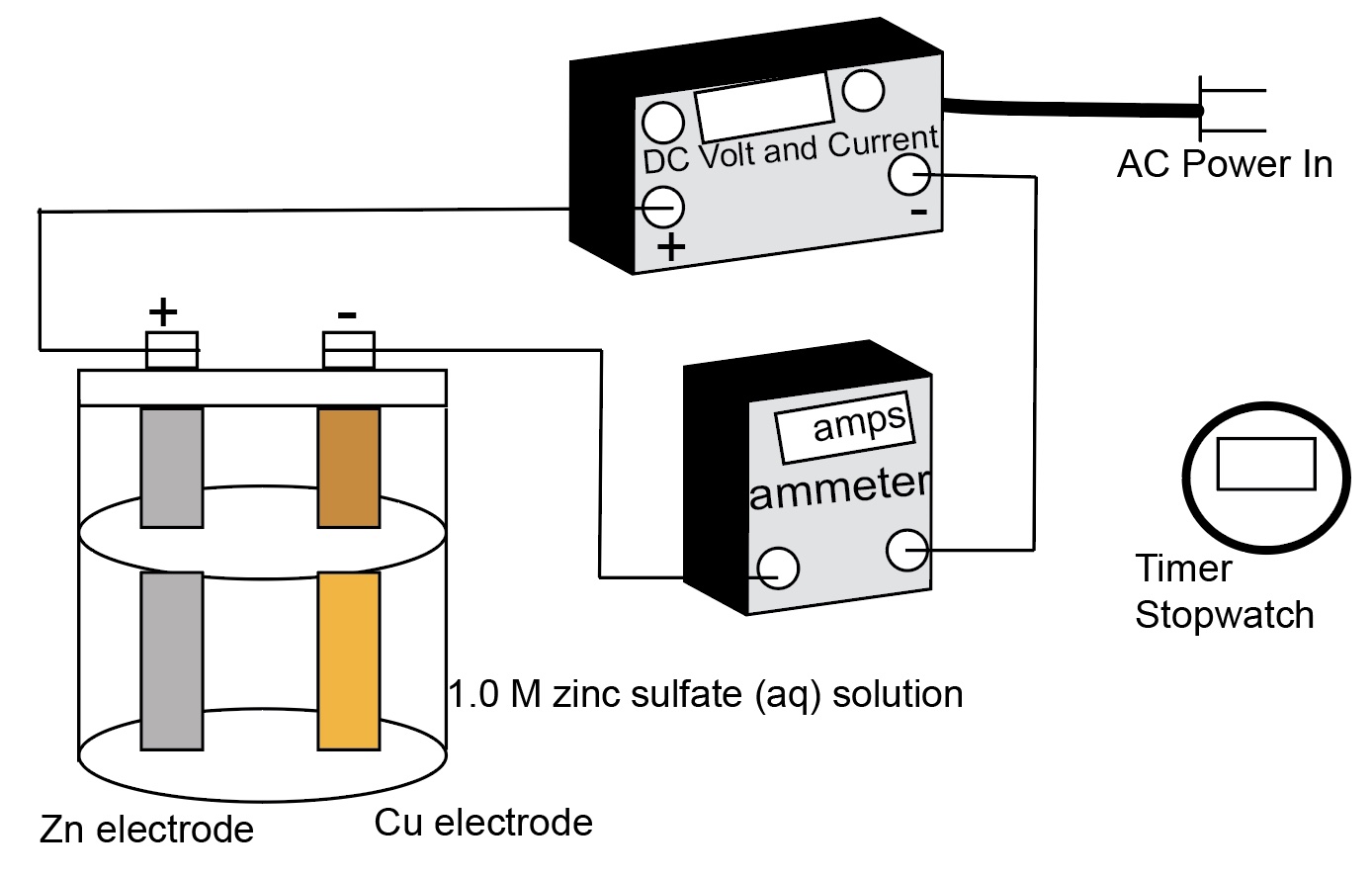
Diagram of a zinc-copper electrolysis cell equipment set-up.
Animations of the reduction and oxidation processes at each electrode show what occurs at the particle level. At the copper electrode each zinc ion gains two electrons and becomes a zinc atom, which plates on the copper electrode.
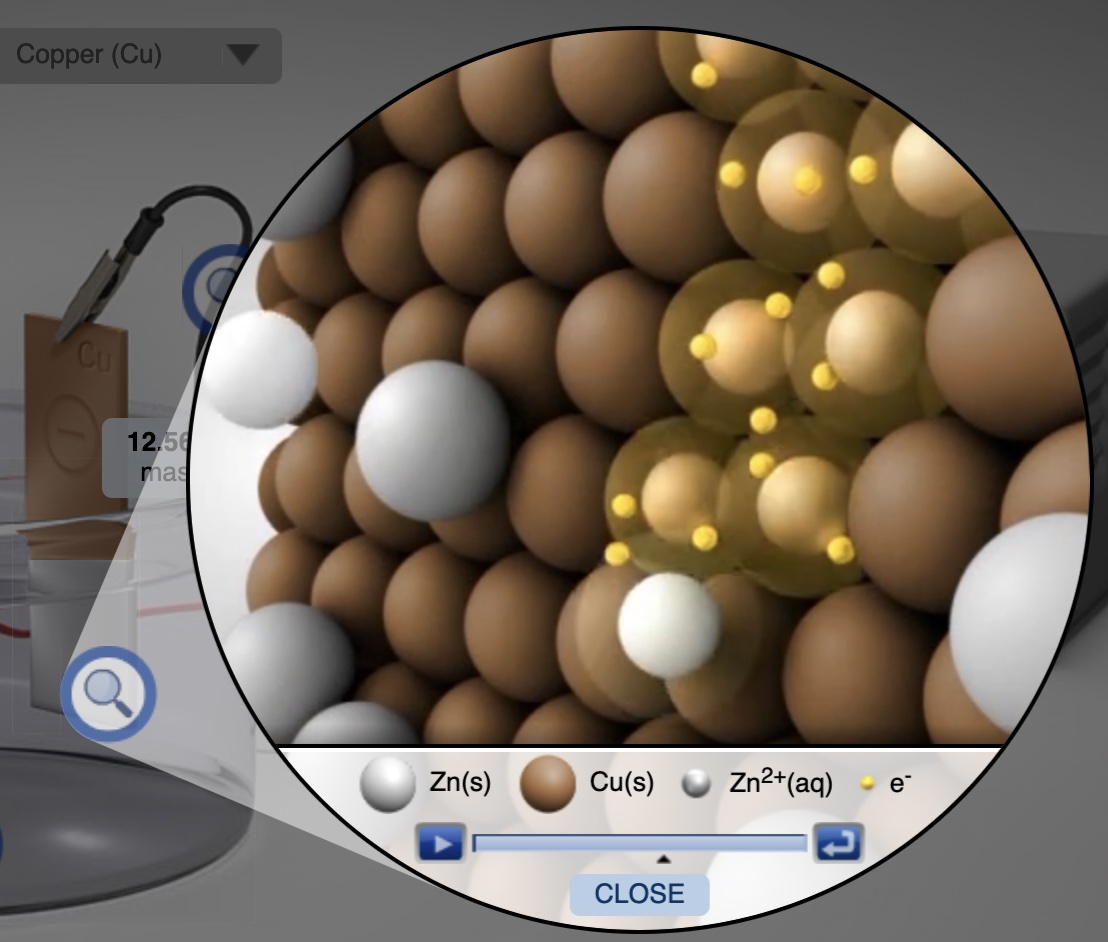
An image from the particle level animation of platting zinc atoms on a copper electrode.
Web-page author: T. Greenbowe, University of Oregon. This page is under construction.
Animation of zinc ions being reduced to zinc metal on the copper electrode. Representation of the reduction process at the particle level.
Student electrolysis cell diagram.
xx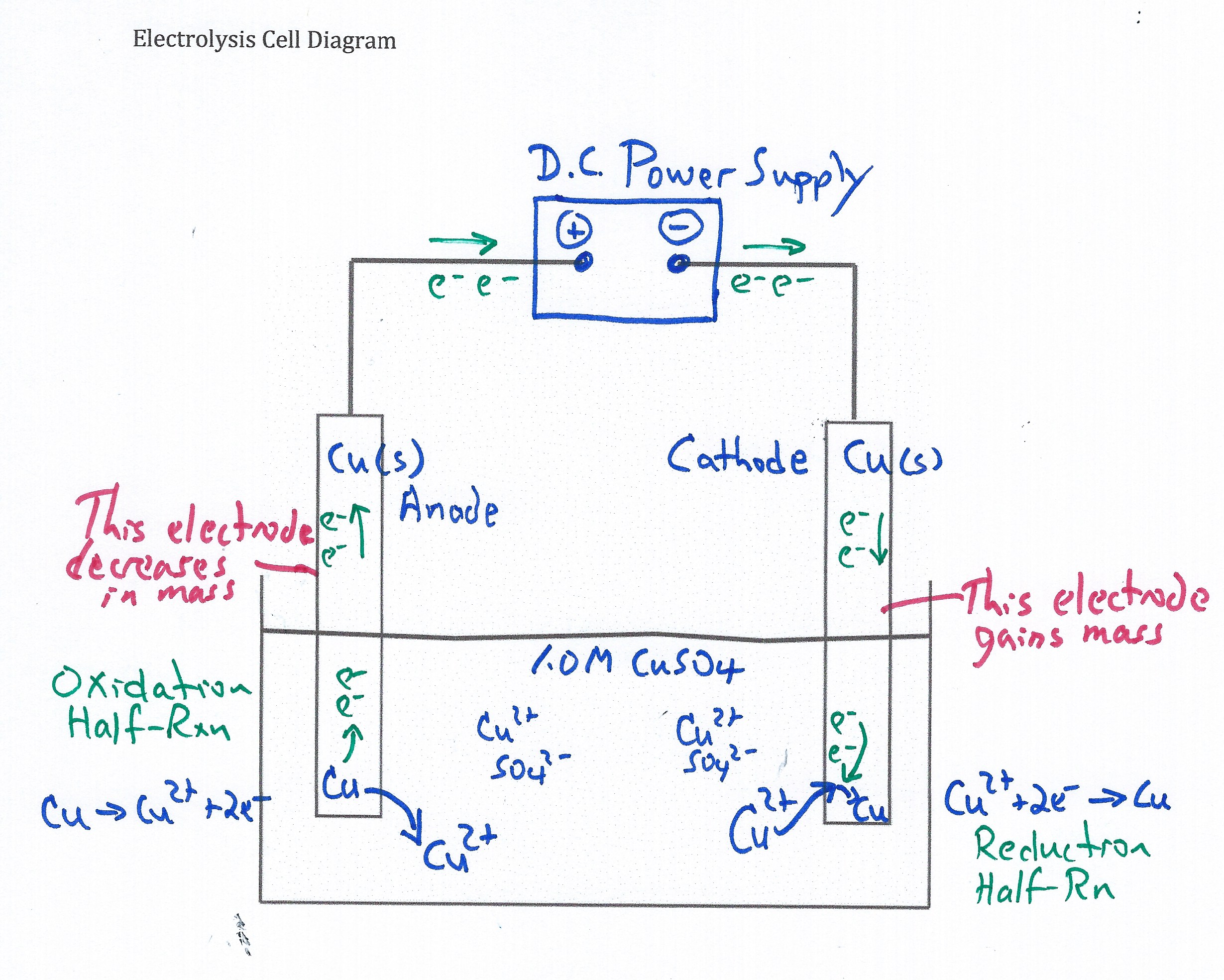
Students complete a diagram of the electrolysis cell.
Curriculum Notes
pair this computer simulation with a demonstration of a real electroylsis cell.

Representing the oxidation and reduction half-reactions at the particle level helps students understand the chemistry involved in electrolysis and makes connections between the symbols and the macroscopic levels (Johnstone, 1993).
Learning Objectives
1. Given a diagram of an electrolytic cell, identify the anode, cathode, direction of which electrons and ions move, the location of the oxidation half-reaction, the location of the reduction half reaction.
2. Given a description or a diagram of an electrolytic cell, write the oxidation half-reaction and the reduction half-reaction.
3. Relate the amount of product(s) generated in an electrolytic cell to the stoichiometry of the reduction half-reaction and to the amount of electrical charge passed in the cell.
4. At the particle level of representation (atom level), show how the number of electrons involved in a single reduction half-reaction, i.e. Zn2+ + 2e- -> Zn, scales up to the mole level: i.e. 1 mole Zn2+ + 2 mole e- -> one mole Zn.
5. Calculate the mass of product produced during electrolysis given the stoichiometry, the amount of electrical current passed in a specific time in the cell.
6. Calculate the quantity of of charged passed in an electrolytic cell, given the stoichiometry, and the amount of electrical current passed in a specific time in the cell.
7. Determine the relationship among coulombs, faradays, time, and reduction-half reaction for an electrolysis cell.
Footnotes
References
Gelder, J.I., Abraham, M.R., Greenbowe, T.J. (2015). “Teaching electrolysis with guided-inquiry.” In Sputnik to Smartphones: A Half-Century of Chemistry Education, M. Orna (ed.) ACS Symposium Series, Volume 1208, pp 141-154. American Chemical Society, Washington, D.C.
Sanger, M. Greenbowe, T. (1997). Common students misconceptions in electrochemistry: Galvanic, electrolytic and concentration cells. Journal of Research in Science Teaching, Volume 34, Issue 4, pages 377–398.
Garnett, P. J., & Treagust, D. F. (1992). Conceptual difficulties experienced by senior high school students of electrochemistry: Electrochemical (galvanic) and electrolytic cells. Journal of Research in Science Teaching, 29(10), 1079-1099.
Penelope Ann Huddle, Margaret Dawn White, and Fiona Rogers, “Using a Teaching Model to Correct Known Misconceptions in Electrochemistry,” J. Chem. Educ., Vol. 77, 2000, 104−110.
Shakhashiri, Bassam Z. (1983) "Galvanizing: Zinc Plating", Chemical Demonstrations, A Handbook for Teachers of Chemistry, v.4, pp. 244-246. U. of Wisconsin Press, Madison, WI.
