Plate zinc metal on copper using electrolysis. Demonstration of a real electrolysis cell accompanied by a computer simulation of an electrolysis cell. Students are given the opportunity to select what metal electrodes to use, where to position the metal electrodes in the cell, what aqueous solution to use, the current to use and the time to push the current through the cell. The electrolysis cell is set-up so that a copper electrode is placed at the cathode where reduction occurs Zn2+(aq) + 2e --> Zn(s) . The current and time on the voltage and current generator are set. The simulations shows the change in mass for the anode and cathode. Animations of ions in the solution ae embedded in the simulation at the particle level shows what occurs at the anode and cathode; plus in the solution and the movement of electrons in the wire.
Web Page Author: T. Greenbowe, University of Oregon. This page is under construction.
  |
http://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/html5/Electro/Electro.php
Greenbowe, T., Abraham, M. Gelder, J., (2016 ). Electrolysis Computer Simulation, Pearson; Hoboken, NJ. If you use the simulation, cite the simulation. The simulation may not be used in any lesson sold for profit.
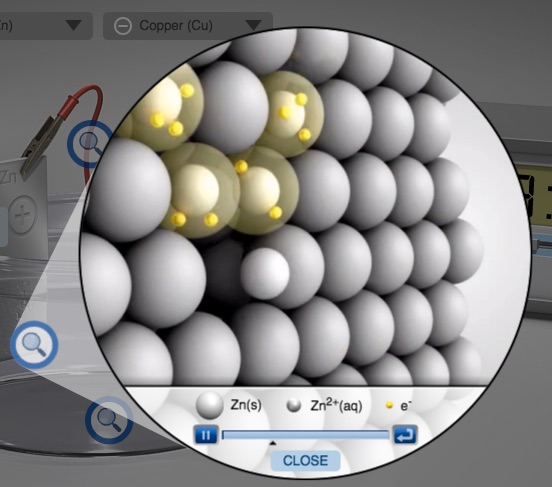 |  |
Particle representation animations of the oxidation half-reaction at the anode and the reduction half-reaction at the cathode. The image menu at the right has a sample of the animation.
This can be an effective demonstration when implemented using active learning. Students are given the opportunity to select what metal electrodes to use, where to position the metal electrodes in the cell, what aqueous solution to use, the current to use and the time to push the current through the cell. Students are given an unmarked diagram of an electrolysis cell and asked to complete the diagram. There is a student activity sheet and a series of "clicker questions" that accompanies this activity.
Representation of the dynamic process that occurs at the cathode as an animation at the particle level
Overview of the Chemistry Associated with this Demonstration
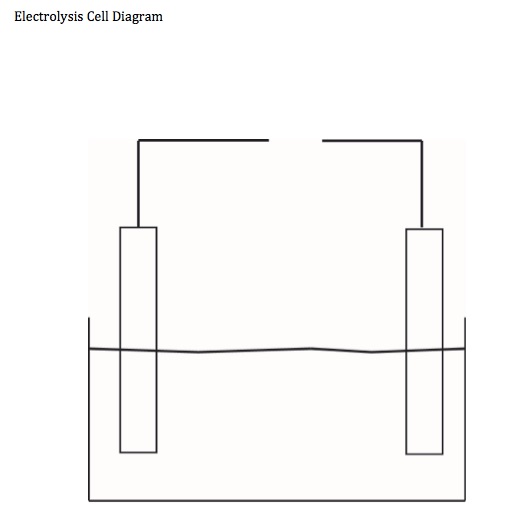
Students are given a blank electrolysis cell diagram and are asked to complete it while observing the demonstration/presentation.
 | 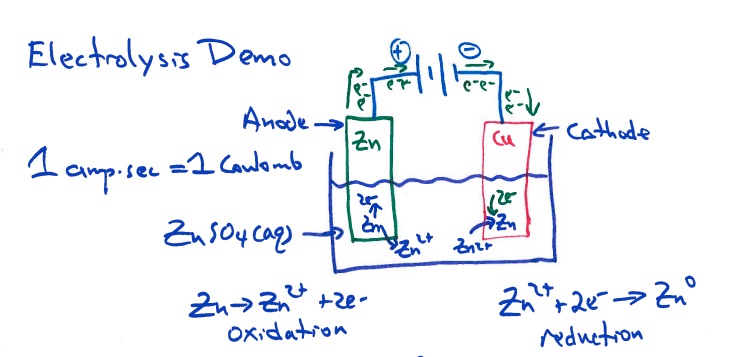 |
cathode Zn2+ + 2e- -> Zn(s) (reduction)
anode Zn(s) -> Zn2+ + 2e- (oxidation)
Target Concepts: It takes two electrons to convert one Zn2+ ion to one Zn atom. It takes six electrons to convert three Zn2+ ion to three Zn atoms. It takes 2 moles electrons to convert one mole of Zn2+ ions to a one mole of Zn atoms. The metal connected to the negative terminal of the battery will serve as the site of a reduction half-reaction. This electrode will have metal plate on it. This electrode is a passive electrode - affords a metal object for electrons to migrate. The oxidation half-reaction and the reduction half-reaction occur simultaneously. Electrons do not pass through the aqueous solution. A mass migration of cations and anions in opposite direction in the aqueous solution constitutes a current. Electrolysis is a dynamic process. A battery supplies the energy needed to cause a "nonspontaneous" or thermodynamic unfavorable chemical reaction to occur.
Facts: 96,500 coulombs represents one mole of electrons involved in electrolysis. One amp second is one coulomb.
Chemicals, equipment, glassware
- copper, zinc, magnesium, stainless steel metals
- copper electrode, zinc electrode, stainless steel electrode
- 0.50 M aqueous copper(II) sulfate solution
- 0.50 M aqueous zinc sulfate solution
- 0.50 M aqueous magnesium sulfate solution
- D.C. power supply
- ammeter
- red and black wires with alligator clips
- timer
- balance
- squirt bottle of water to rinse electrodes
- paper towels
- hair dryer
Procedure
Prerequisite Knowledge: First, ask students if a reaction will occur when zinc metal is placed in aqueous copper(II) sulphate? ask if a reaction occurs when copper metal is placed in aqueous zinc sulphate? Demonstrate placing a piece of copper metal in aqueous zinc sulfate and placing a piece of zinc metal in aqueous copper(II) sulfate.
This will establish that copper metal will not react with zinc sulfate. Zinc is a more active metal compared to copper.
Second, show an animation of a simple direct current circuit with a battery, a bulb and switch. This will establish that electrons are pushed out of the negative terminal of the battery and pulled into the positive terminal of the battery.
Third, remind students what is present in an ionic aqueous solution, water molecules, cations, and anions. Remind students of the conductivity apparatus and that an aqueous zinc sulphate solution is a strong electrolyte - meaning cations and anions are present.
Have students work with a blank electrolysis cell diagram. The goal of this electrolysis demonstration is to plate zinc metal on copper metal. Ask students which metal should be connected to the negative terminal of the battery and which metal should be connected to the positive terminal of the battery. Have students make the prediction. Show students the shiny electrodes. If possible obtain the mass of each electrode.
Show students the two diagrams of the electrolysis experiment set-up: One diagram has the components represented and identified. The second diagram uses the "shorthand" physics symbols notations for the battery, ammeter, voltmeter, switch and electrolysis cell containing the metal electrodes and aqueous solution.
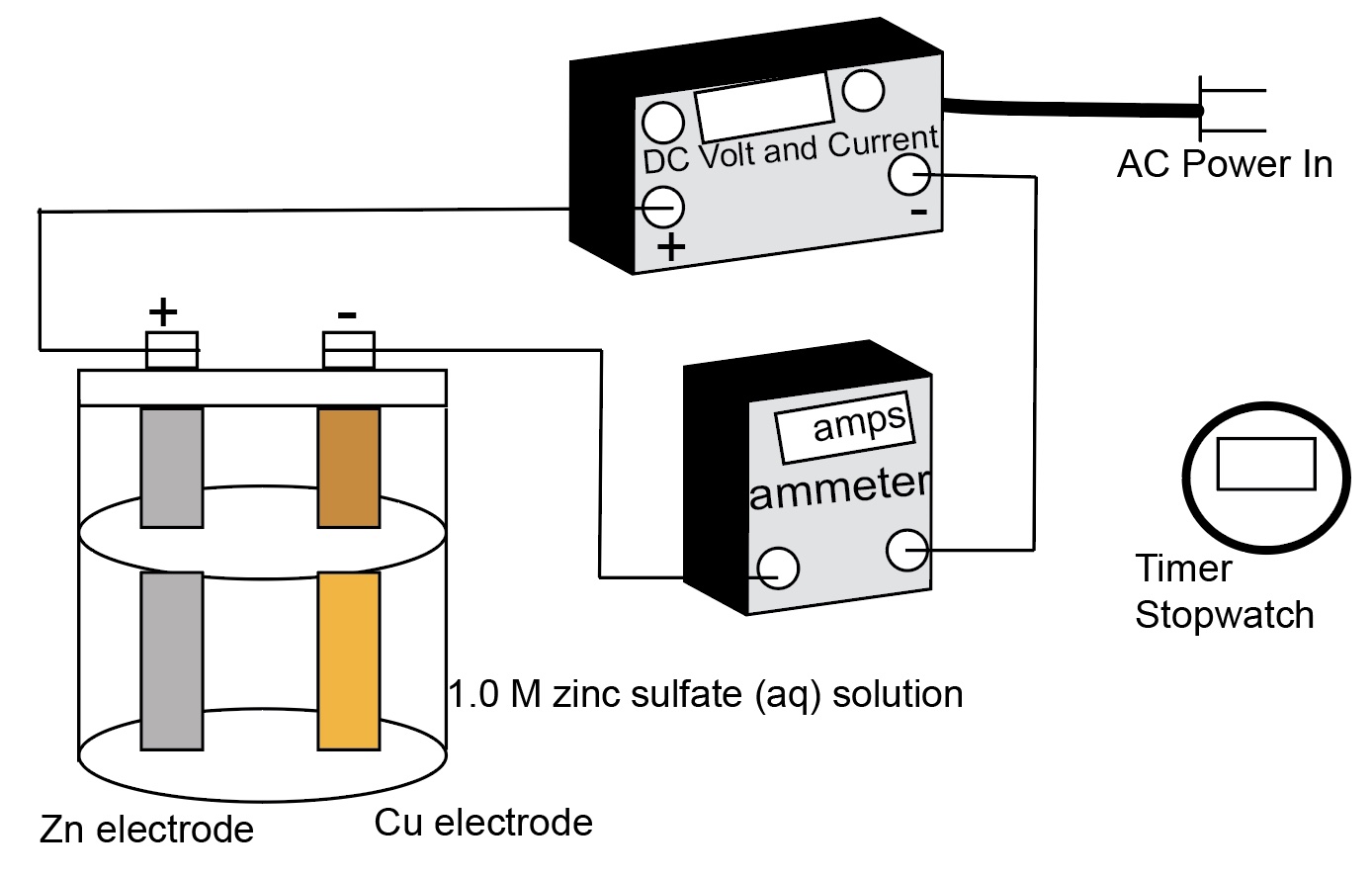
Connect the electrodes to in the electrolysis cell. Note which metal is connected to the negative terminal using a black wire and which is connected to the positive terminal using a red wire. Make sure an ammeter is connected in series to the circuit. Add aqueous zinc sulphate to the cell. Set up the same cell using the electrolysis computer simulation. Note the mass of each electrode.

Turn on the DC voltage generator Record the current and keep track of the time. When zinc is visible on the copper electrode stop the electrolysis. Have the students identify which electrode is having the zinc metal plate on to it. Turn on the real electrolysis cell. Let the cell run.
Turn students' attention to the electrolysis simulation. Note the initial mass of each electrode. Set the amperage and time on the simulation, record the current and time, and turn on the simulation. Click-on the "microscopic" button. Click-on the magnifying glass near the anode. Have students observe the animation and have students write the half-reaction occurring at this electrode. Do not tell students the half-reaction, let the students figure it out. Click-on the magnifying glass near the cathode. Have students observe the animation and have students write the half-reaction occurring at this electrode. Do not tell students the half-reaction, let the students figure it out. Point out that it takes two electrons to reduce one Zn2+ ion to one Zn atom. Click-on the magnifying glass centered in the solution. Ask the students to observe what occurs in the solution. Next, draw students attention to the mass of the electrodes. The anode is decreasing in mass and the cathode is increasing in mass. Ask students to explain why this occurs. Students should note that the decrease in mass of the anode equals the increase in mass at the cathode.
Turn off the real electrolysis cell. Ask students to predict which electrode decreases in mass and which electrode increases in mass. Remove the electrodes from the cell and carefully dry the electrodes, measure the mass of each electrode. Students should note that the decrease in mass of the anode equals the increase in mass at the cathode.
Note: This demonstration is a modification of "Galvanizing: Zinc Plating," from Bassam Z. Shakhashiri's Chemical Demonstrations, A Handbook for Teachers of Chemistry, v.4, (U. of Wisconsin, 1983) pp. 244-246.
Student Difficulties (Misconceptions) with Electrolysis. The first sentence is the misconception or student difficulty. The second sentence is a statement of the correct science.
1. When identical metal electrodes are used in an electrolysis cell, the same reactions occurs at both electrodes and the products are the same at both electrodes.
When identical metal electrodes are used in an electrolysis cell, the electrode connected to the positive terminal of a D.C. (direct current) power supply or battery serves as the anode (site of oxidation Cu --> Cu2+ + 2e-) and the electrode connected to the negative terminal of a D.C. (direct current) power supply serves as the cathode (site of reduction: Cu2+ + 2e- __> Cu ).
2. The power source used in an electrolytic cell pulls electrons in at the negative terminal and pushes electrons out at the positive terminal. When a direct current power source is used in an electrolysis cell, electrons are forced out of the negative terminal and pulled in at the positive terminal.
3. During electrolysis, electrons move through electrolyte solution by being attracted to positive ions in the solution. During electrolysis, there is a mass migration of cations and anions in opposite direction in the electrolytic solution. The cations migrate toward the cathode and the anions migrate toward the anode. Electrons do not travel from one electrode to the other through an electrolyte solution.
4. During electrolysis, no reaction will occur if two inert electrodes are used. While the material of an inert electrode does not react, an inert electrode serves as a conduit for the electrons to interact with species in the electrolyte solution.
5. In electrolytic cells, oxidation occurs at the cathode and reduction occurs at the anode. In all electrochemical cells, oxidation occurs at the anode and reduction occurs at the cathode.
6. In electrolytic cells, water is unreactive and does not undergo oxidation and reduction. In electrolytic cells, of there are species present that are more reactive compared to water. Water can undergo an oxidation-reduction reaction - the electrolysis of water will generate hydrogen gas and oxygen gas.
7. When writing the chemical reaction that occurs in an electrolytic cell, the half-cell reactions are reversed prior to combining them. When writing the chemical reaction that occurs in an electrolytic cell, the half-cell reactions are combined.
8. The calculated cell potentials in electrolytic cells can be positive. Since the reactions involved in electrolysis cells are always "non-spontaneous", the calculated cell potentials in electrolytic cells are negative.
9. In electrolytic cells, the direction of the applied voltage has no effect on the reaction or the site of the anode and cathode. In electrolytic cells, the direction of the applied voltage determines the site of the anode and cathode.
10. The number of electrons transferred in an electrolysis cell is proportional to the emf or volts, i.e. more electrons transferred means a higher voltage. The number of electrons transferred in an electrolysis cell is proportional to the current (amps) and time, i.e. the higher the amperage for a longer time means more electrons transferred.
The number of electrons transferred in an electrochemical cell is the charge in units of Coulombs. The current is the number of electrons that passes through an electrochemcial cell in a given period of time. 1 Coulomb per second is 1 ampere. 1 amp = 1 Coulomb/sec
Learning Objectives
1. Given a diagram of an electrolytic cell, identify the anode, cathode, direction of which electrons and ions move, the location of the oxidation half-reaction, the location of the reduction half reaction.
2. Given a description or a diagram of an electrolytic cell, write the oxidation half-reaction and the reduction half-reaction.
3. Relate the amount of product(s) generated in an electrolytic cell to the stoichiometry of the reduction half-reaction and to the amount of electrical charge passed in the cell.
4. At the particle level of representation (atom level), show how the number of electrons involved in a single reduction half-reaction, i.e. Zn2+ + 2e- -> Zn, scales up to the mole level: i.e. 1 mole Zn2+ + 2 mole e- -> one mole Zn.
5. Calculate the quantity of of charged passed in an electrolytic cell, given the stoichiometry, and the amount of electrical current passed in a specific time in the cell.
6. Calculate the mass of product produced during electrolysis given the stoichiometry, the amount of electrical current passed in a specific time in the cell.
7. Determine the relationship among coulombs, Faradays, time, and reduction-half reaction for an electrolysis cell.
AP Chem Learning Objectives The student can
LO 3.12: make qualitative or quantitative predictions about electrolytic cells based on half-cell reactions and potentials and/or Faraday's laws.
LO 3.13: analyze data regarding electrolytic cells to identify properties of the underlying REDOX reactions.
LO 5.15 is able to explain how the application of an external energy source (power source) can be used to cause processes that are not thermodynamically favorable to become more favorable (i.e. to occur).
Pre-Requisite Knowledge
1. Oxidation-reduction reaction reactions involve a transfer of electrons. Usually, there are two species involved in a REDOX reaction - both species change oxidation states - one increases its oxidation state, the other decreases its oxidation state.
2. Galvanic cells convert chemival energy into electrical energy.
3. Physics: voltage and current; the function of Direct Current (DC) power supplies; how batteries push electrons in a circuit
References
Bong A. Y. L. and Lee T. T., (2016), Form four students’misconceptions in electrolysis of molten compounds and aqueous solutions, Asia-Pac. Forum Sci. Learn. Teach., 17(1), 8.
Gelder, J.I., Abraham, M.R., Greenbowe, T.J. (2015). “Teaching electrolysis with guided-inquiry.” In Sputnik to Smartphones: A Half-Century of Chemistry Education, M. Orna (ed.) ACS Symposium Series, Volume 1208, pp 141-154. American Chemical Society, Washington, D.C.
Sanger, M. Greenbowe, T. (1997). Common students misconceptions in electrochemistry: Galvanic, electrolytic and concentration cells. Journal of Research in Science Teaching, Volume 34, Issue 4, pages 377–398.
Garnett, P. J., & Treagust, D. F. (1992). Conceptual difficulties experienced by senior high school students of electrochemistry: Electrochemical (galvanic) and electrolytic cells. Journal of Research in Science Teaching, 29(10), 1079-1099.
Loh A. S. L., Subramaniam R. and Tan K. C. D., (2014), Exploring students’ understanding of electrochemical cells using an enhanced two-tier diagnostic instrument, Res. Sci.Technol. Educ., 32(3), 229–250.
Ozkaya, A.R., Uce, M., Sahin, M. (2003). Prospective teachers’ conceptual understanding of electrochemistry: galvanic and electrolytic cells. University Chemistry Education, 7, 1-12.
Penelope Ann Huddle, Margaret Dawn White, and Fiona Rogers, “Using a Teaching Model to Correct Known Misconceptions in Electrochemistry,” J. Chem. Educ., Vol. 77, 2000, 104−110.
Sia D. T., Treagust D. F. and Chandrasegaran A. L., (2012), High school students’ proficiency and confidence levels in displaying their understanding of basic electrolysis concepts,
Int. J. Sci. Math. Educ., 10(6), 1325–1345.
Shakhashiri, Bassam Z. (1983) "Galvanizing: Zinc Plating", Chemical Demonstrations, A Handbook for Teachers of Chemistry, v.4, pp. 244-246. U. of Wisconsin Press, Madison, WI.
Johnstone, A. H. 1993. The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9): 701-705.
