It is important to note that the kinetics system we are working with is designed to be a demonstration of concepts of kinetics and not a kinetics experiment.
Qualitative Approach (Option 1):
The purpose of this demonstration is to provide visual evidence of the influence of concentration on the time it takes for a reaction to go to completion. Two colorless solutions are mixed and after a brief induction period, the resultant clear solution suddenly (abruptly) turns to a blue-black color. Changing the concentration of reactants in this clock reaction changes the induction period. For option 1, the temperature of all solutions is at room temperature and only the concentration of potassium iodide changes. Students need to identify the independent, dependent, and control variables in this demonstration. This demonstration works best if three reactions are run simultaneously.
- Beaker System A: [H2O2] = 0.045 M; [KI] = 0.100 M
- Beaker System B: [H2O2] = 0.045 M; [KI] = 0.050 M
- Beaker System C: [H2O2] = 0.045 M; [KI] = 0.025 M
The concentrations of the other reactants remain constant. ([H2SO4] = 0.10 M; [Na2S2O3] = 0.0028 M).
Student Worksheet:
Independent Variable _________
Dependent Variable ____________
Control Variable _______________
| Experiment | [H2O2], M | [KI], M | Reaction Time | Rate of Reaction |
| A | 0.045 | 0.100 | ||
| B | 0.045 | 0.050 | ||
| C | 0.045 | 0.025 |
This web page is under construction. Web page author: T. Greenbowe, University of Oregon, Eugene, Oregon.
Quantitative Approach Option 2:
Quantitative Approach Option 3: The reaction is first run with specific concentration of reactants and the induction period is timed. Then the same reaction is run with the concentration of potassium iodide halved and the induction period is timed again.
The reaction is first run with a certain concentration of reactants and the induction period is timed. Then the same reaction is run with the concentration of potassium iodide halved and the induction period is timed again. Lastly the hydrogen peroxide concentration is halved and the the induction period is timed. The results of the kinetics experiment can be used to derive the order of dependency of the rate on the concentration of iodide ion and hydrogen peroxide, i.e. the rate law, Rate = k [H2O2]x [KI]y
Student Worksheet:
Independent Variable _________
Dependent Variable ____________
Control Variable _______________
Experiment [H2O2], M [KI], MReaction Time Rate of Reaction
| Experiment | [H2O2], M | [KI], M | Reaction Time | Rate of Reaction |
| H | 0.045 | 0.100 | ||
| I | 0.045 | 0.050 | ||
| J | 0.0225 | 0.100 |
Curriculum Notes
This demonstration works well when presenting a unit on kinetics: the rates of reaction and the method of initial rates. The rate of reaction is how fast or slow a reaction occurs relative to a standard. The greater the rate of reaction, the less time it takes for the reaction to go to completion, i.e. the less time it takes for reactants to be converted to products. When focusing students' attention on the role of concentration of reactants with respect to the rate of reaction it is important to discuss and or make the connection to collision theory. The collision model assumes that molecules must collide in order for a reaction to occur. The more molecules present, the more chance for frequent "effective" collisions, the more often a reaction occurs. Reaction rate is proportional to the number of effective collisions, which depends on the concentration of the reactants. Inclusion of several picture diagrams or "molecular scenes" as well as showing one of our computer animations comparing two systems with different initial concentration of reactants is recommended. The reaction rate of the system with double the initial concentration is twice that of the other. However, the time it takes for the reaction to go to completion of the system with double the initial concentration is half the time. This should help students visualize what is occurring on the molecular level as well as make note of the inverse relationship between reaction rate and time.
Students have a difficult time with the concept of a greater reaction rate taking less time.
Interactive lecture slides designed to accompany this demonstration are available.
A computer simulation can accompany this demonstration
Iodine Clock Computer Simulation at UO
http://pages.uoregon.edu/tgreenbo/iodine_clock.html
The computer simulation URL is also available at
Prof. John Gelder's Department of Chemistry, Oklahoma State University web site
"iodine Clock Computer Simulation"
http://introchem.chem.okstate.edu/DCICLA/iodine_clock.html
An excellent guided-inquiry resource for an alternative approach to Iodine Clock Reaction using potassium iodate and sodium bisulfite available from Flinn Scientific ©2016. This is a guided inquiry experiment.
https://www.flinnsci.com/api/library/Download/1fec5df4c5a64a7f98df4730ab...
“The Order of Reaction” experiment in Kinetics, Volume 14 in the Flinn ChemTopic™ Labs series; Cesa, I., Editor; Flinn Scientific: Batavia, IL (2003).
One day of lead time is required for this project.
Discussion
While this demonstration is effective, the overall understanding of the reaction mechanism is complicated for students. The thiosulfate ions quickly consume the triiodide ions. The blue-black color does not appear until all of the thiosulfate ions are consumed. The thiosulfate ions are the limiting reactant. The rate of reaction is first-order in potassium iodine. For the qualitative option, the details of the mechanism are not revealed to the students in order to have the students focus on the kinetics concepts of changing the concentration of one reactant versus time or reaction.
For the entire explanation of this demo see Bassam Shakhashiri.Chemical Demonstrations: A Handbook for Teachers of Chemistry, Vol. 4, pp 42-43. U. of Wisconsin: (1992). The following is a excerpt from this volume.
" The sudden change from colorless to deep blue-black solutions in this demonstration can be explained with the following sequence of equations:
- 3 I-(aq) + H2O2 + 2 H+(aq) ==> I3-(aq) + 2 H2O(l)
- I3-(aq) + 2 S2O3 2-(aq) ==> 3 I-(aq) + S4O6 2-(aq)
- 2 I3- (aq) + starch ==> starch-I5- complex + I-(aq)
The first equation indicates that, in an acidic solution, iodide ions are oxidized by hydrogen peroxide to triiodide ions. These triiodide ions are reduced back to iodide ions by thiosulfate ions, as indicated in equation 2. This reaction is much faster than the reaction of equation 1; it consumes triiodide ions as fast as they are formed. This prevents any readily apparent reaction of equation 3. However, after all the thiosulfate ions have been consumed by the reaction of equation 2, triiodide ions react with starch to form the blue starch-pentaiodide complex ." The "A" beakers contain sodium thiosulfate, potassium iodide, and a little bit of starch. The "B" beakers contain hydrogen peroxide and sulfuric acid.
- Reaction 1: [H2O2 ] = 0.045 M; [KI] = 0.050 M
- Reaction 2: [H2O2 ] = 0.045 M; [KI] = 0.025 M
- Reaction 3: [H2O2 ] = 0.0225 M; [KI] = 0.050 M
The concentrations of the other reactants remain constant. ([H2SO4] = 0.10 M; [Na2S2O3] = 0.0028 M). By comparing the length of the induction periods, conclusions can be drawn regarding the dependence of the rate of reaction on the concentration of reactants.
Materials
- Three 600 mL beakers containing clear solutions labeled "1A", "2A", and "3A". (See "Discussion" section for contents of the beakers.)
- Three 400 mL beakers containing clear solutions labeled "1B", "2B", and "3B". (See "Discussion" section for contents of the beakers.)
- Three glass stirring rods.
- A clock or watch for timing the reactions.
Procedure
Option 1
Have 3 students, safety goggles on, pour the solutions of B into A simultaneously. Begin timing. Stir vigorously
Option 2
- Pour the contents of beaker 1B into the beaker labeled 1A. Begin timing. Stir vigorously until the solution turns blue. Record the time that elapsed.
- Pour the contents of beaker 2B into the beaker labeled 2A. Begin timing. Stir vigorously until the solution turns blue. Record the time that elapsed.
- Pour the contents of beaker 3B into the beaker labeled 3A. Begin timing. Stir vigorously until the solution turns blue. Record the time that elapsed.
- This demonstration takes about twelve minutes to present.
Safety Precautions
Avoid getting any of the solutions on you. If you do get chemicals on your skin, wash thoroughly with soap and water. If you get chemicals in your eyes, flush with water for 15 minutes and seek medical attention.
Footnotes
Prep. Notes
For preparation, refer to Bassam Shakhashiri. Chemical Demonstrations: A Handbook for Teachers of Chemistry, Vol. 4, p. 39. U. of Wisconsin: (1992). Modify the instructions for Procedure B as follows:
- Prepare two of the "Beaker 2" solutions. These are our beakers "1A" and "3A".
- Prepare one of the "Beaker 4" solutions. This is our beaker "2A".
- Our beakers "1B" and "2B" contain Shakashiri's acidified peroxide solution, "B-3".
- Our beaker "2C" is like Shakashiri's "B-3", but with half of the concentration of hydrogen peroxide
Animation of the effect of concentration on rate of reaction - particle behavior
Consider the simple reaction A + B -> C
Run the simulation "rate of reaction" AACT. Choose increase concentration, record your observations of the initial and final number of particles of A, B and C, as well as the time. Choose decrease concentration, record your observations.
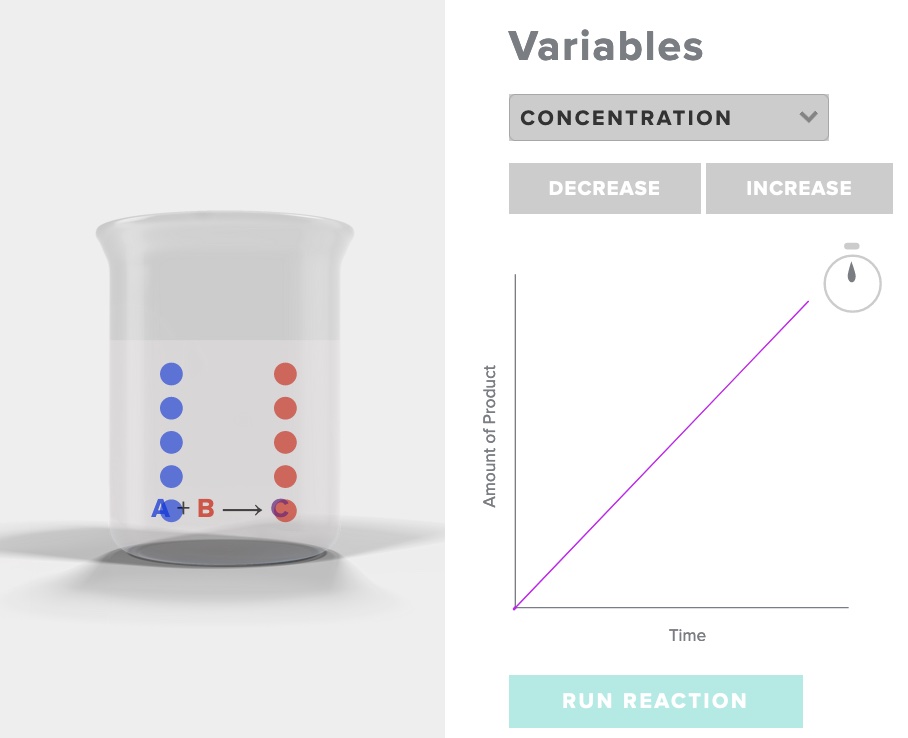
After interacting the the AACT simulation "Reaction Rates" explain why the rate of reaction is affected by a change in concentration with specific reference to the behavior of particles in three situations - concentration [A], concentration 1/2[A], and concentration 2[A].
References
1. Bassam Shakhashiri. (1992). Chemical Demonstrations: A Handbook for Teachers of Chemistry, Vol. 4, pp 42-43. U. of Wisconsin.
