Three commercial glow sticks are started. One is at room temperature (23°C). One is immersed in a hot water bath (80°C). It glows more brightly compared to the light stick at room temperature. The third light stick is immersed in an ice water bath (0.0°C) or in liquid nitrogen. It glows dimly. This series of comparisons of the amount of light being given off, which is proportional to the rate of reaction, illustrates how temperature influences rates of reaction.
Student Observation Table
Temperature | Relative Amount of Light | Relative Reaction Rate |
Hot water (80°C) |
|
|
Room temperature (23°C) |
|
|
Cold water (0.0°C) |
|
|

Videos:
http://www.youtube.com/watch?v=fBtCTcRYEUk
http://www.youtube.com/watch?v=RH8TcwsHsvc
A set of Power Point Slides is available to accompany this demonstration.
Allow about 5 minutes for this demonstration.
This page is under construction. Web page author: T. Greenbowe, University of Oregon
Materials
- 3 light sticks with strings tied to them
- a beaker containing hot water
- a beaker
- a beaker containing ice and water
Procedure
- Allow about 5 minutes for this demonstration.
- Dim the lights.
- Snap and shake the three light sticks so that they begin to glow.
- Place one of the light sticks in the beaker containing ice water and another in the beaker containing the hot water. The third light stick serves as a control. Leave the light sticks in their respective beakers for about thirty seconds.
- Withdraw the light sticks from their beakers using the attached strings. Compare their relative brightness. The light stick that was immersed in the hot water should be brighter than the control stick at room temperature and the stick that was immersed in the ice water should be dimmer than the control stick.
Safety
- The demonstrator will be working around very hot water in conditions of impaired visibility (dim light). Be careful, hot water can cause severe burns.
- Vapor pressure can build up in the heated light stick and the heat weakens the plastic. Heat the water to 80°C not to the boiling point. Light sticks have been known to burst under conditions of hot temperatures above 80°C. The demonstrator must wear goggles to protect eyes. Students should be at least 3 meters away from the demonstration area.
This demonstration is usually performed when collision theory and factors that influences the rate of reactions (temperature) are being discussed.
This demonstration shows the effect of temperature on reaction rate. Raising the temperature of a reaction mixture results in both more frequent and more energetic molecular collisions. Increasing the temperature increases the average kinetic energy of the molecules. At higher temperatures molecular collisions are more likely to have enough energy to form products. This increases the rate of reaction. At lower temperatures, there are fewer collisions and most of the collisions that do occur between reactants do not have sufficient energy to form products. Because at any temperature in a gas, liquid, or solution, there is a distribution of molecular speeds, some molecules will have sufficient energy that when they collide a reaction will occur. At reasonably cold temperatures reactions occur but there are fewer effective reactions compared to the system at higher energy.
In a chemoluminescent reaction such as this one, at elevated temperature, the increased reaction rate can be perceived as increased brightness of the light being given off by the light stick. At cold temperatures, the light stick glows less brightly because in a given time period, fewer reactant molecules are colliding with sufficient energy to form the products.
The chemical reaction in a light stick usually involves several different steps. A typical commercial light stick has a thin walled-glass ampule containing hydrogen peroxide solution floating in a solution of a phenyl oxalate ester with a fluorescent dye.
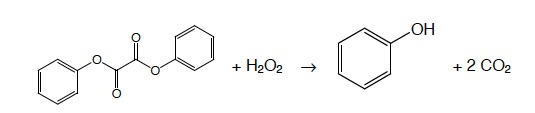
During the reaction, an intermediate is produced which transfers energy to the fluorescent dye molecule. When the dye molecule absorbs energy, the energy is used to raise electrons to an excited state. When the dye molecule returns to the ground state, the excited electrons return to the ground state and energy in the form of light is emitted. E = hv Photons are emitted.
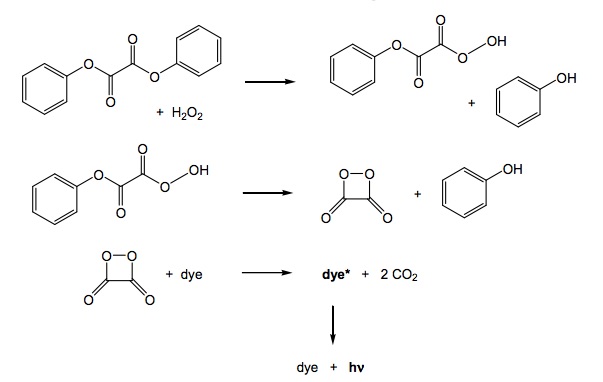
Here's the sequence of events that occur when the two solutions are combined:
- The hydrogen peroxide oxidizes the phenyl oxalate ester, resulting in a chemical called phenol and an unstable peroxyacid ester.
- The unstable peroxyacid ester decomposes, resulting in additional phenol and a cyclic peroxy compound.
- The cyclic peroxy compound decomposes to carbon dioxide.
- This decomposition releases energy to the dye.
- The electrons in the dye atoms jump to a higher level, then fall back down, releasing energy in the form of light.
Extensions of the demonstration
Using a light meter in a darkened room, the activation energy can be measured by plotting ln(light intensity) vs. 1/T, T = temperature. The activation energy is approximately 56.4kJ/mole.
Discuss the effect that temperature has on the rate of chemical reactions. Discuss how the energy of activation barrier is overcome. Ask students which light stick will use up the reactants first. Discuss the concept that the intensity of light is proportional to the reaction rate. The faster the rate of reaction, the sooner all the reactants are consumed and the reaction ceases.
Making Connections to Particle Behavior
The influence of temperature on rate of reaction - particle behavior - Animation
Consider the simple reaction A + B -> C
Run the simulation "rate of reaction" AACT. Choose increase temperature, record your observations of the initial and final number of particles of A, B and C, as well as the time. Choose decrease temperature, record your observations.
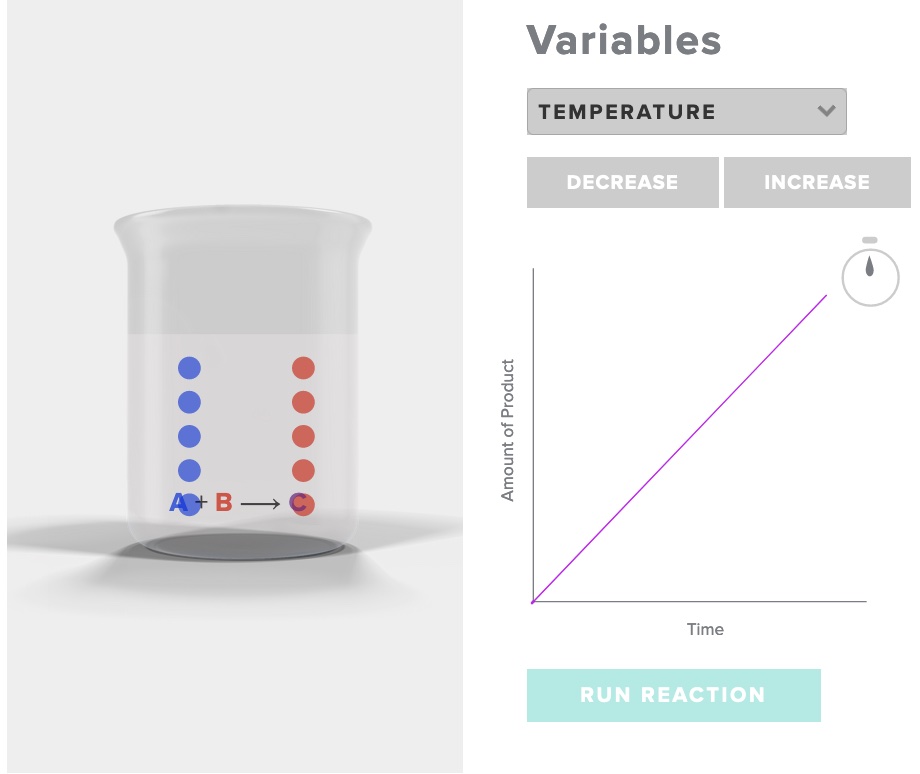
After interacting the the AACT simulation "Reaction Rates" explain why the rate of reaction is affected by a change in temperature with specific reference to the behavior of particles in three situations - temperature T1, temperature T2, and temperature T3,
Learning Outcomes/Objectives
1. An increase in temperature increases the reactions rate. A decrease in temperature decreases the reaction rate.
2. An increase in temperature increases the the average kinetic energy of the molecules, which involves increases the average speed of the molecules.
3. Temperature affects the reaction rate by influencing collision energy and thus, the fraction of collisions with energy exceeding the activation energy.
4. A reaction mechanism consists of a series of steps at the molecular level. Adding up the steps yields the overall overall reaction.
References
1 Bassam Z. Shakashiri, Lightsticks (2.2). Chemical Demonstrations, A Handbook for Teachers of Chemistry, volume.1 (Madison: The University of Wisconsin Press, 1983) pp. 146-152.
2. Bassam Z Shakhashiri, Lloyd G. Williams, Glen E. Dirreen, and Ann Francis, “Cool-Light Chemiluminescence,” J. Chem. Educ., Vol. 58, 1981, 70–72. The dependence of reaction rates on temperature is demonstrated with chemiluminescent light sticks.
3. http://pubs.acs.org/cen/whatstuff/stuff/7703scit4.html (C&EN “What’s That Stuff?”)
