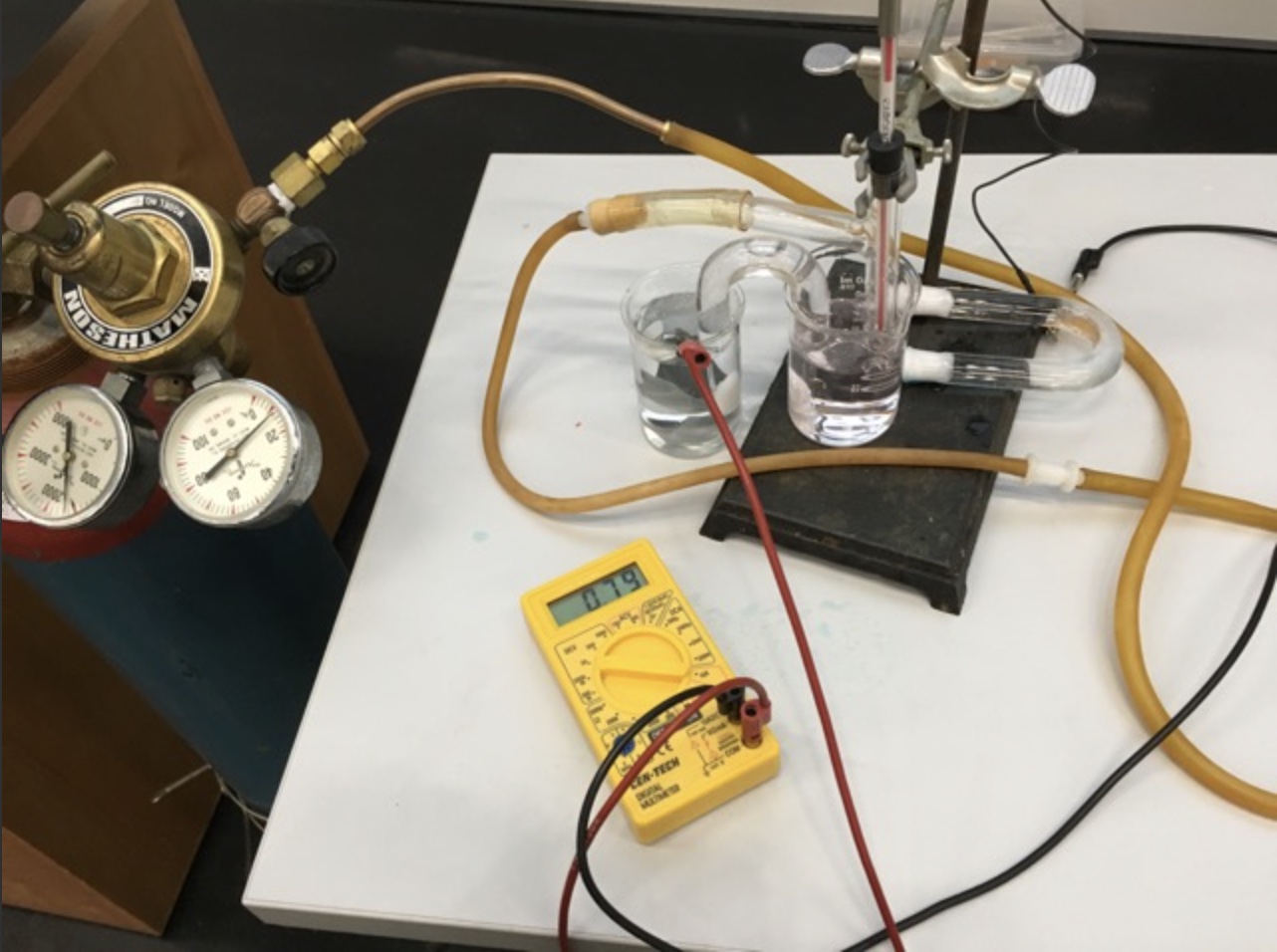
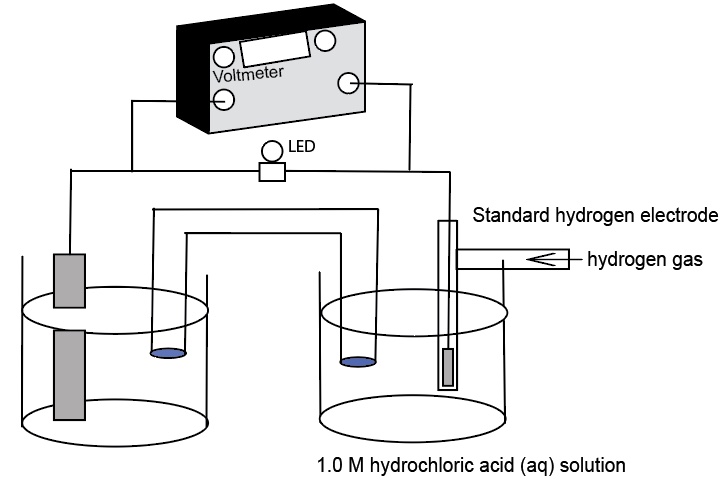
A Standard Hydrogen Electrode (SHE) is used to compare the force of the flow of electrons in an electrochemical cell - electromotive force, emf, in units of volts. The SHE is selected as a standard to which all other half-cells are measured. This demonstration uses a real standard hydrogen electrode and an AACT Voltaic Cell simulation. The real SHE consists of a glass T-tube with entry for hydrogen gas and a platinum wire electrode. At the end of the platinum wire is a thin cuboid of platinum with platinum black. The glass apparatus is placed in 1.0 M HCl(aq) and the hydrogen gas is bubble over the Pt electrode with a catalyst and into the solution. The SHE is used to show the E°cell generated helps to determine the standard reduction potentials of two half-reactions:
Zn2++ 2e- -> Zn E° = -0.76 V and Cu2++ 2e- -> Cu E° = +0.34 V.
A H2(g) tank serves as the source of H2(g) being bubbled into the Standard Hydrogen Electrode (SHE) in 1.00 M HCl(aq) at 25°C ,1.00 atm pressure, near sea level. The reference half-cell is the reduction of hydrogen ions to hydrogen gas
2H+(aq) +2e- -> H2(g, 1 atm) E° = 0.0 V
This occurs when the SHE is acting as a cathode, when it is attached to a more active metal such as zinc.
When the SHE is attached to a less active metal such as copper, the SHE acts as an anode and the oxidation half-reaction is
H2(g, 1 atm) -> 2H+(aq) +2e- E° = 0.0 V
The SHE half-cell reaction can either be 2H+(aq) + 2e- --> H2(g) (an oxidation half-reaction, occurs at the anode)
or the following half-reaction at the SHE electrode when it serves as a cathode
H2(g) --> 2H+(aq) + 2e- (a reduction half-reaction, occurs at the cathode)
 |  |
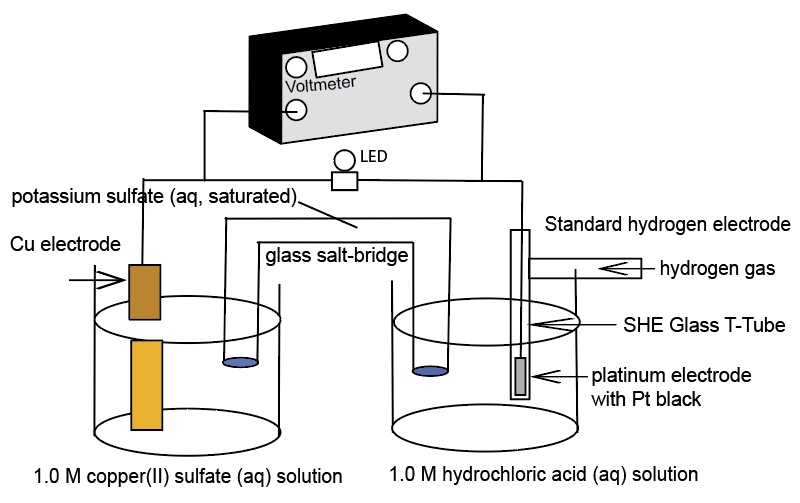
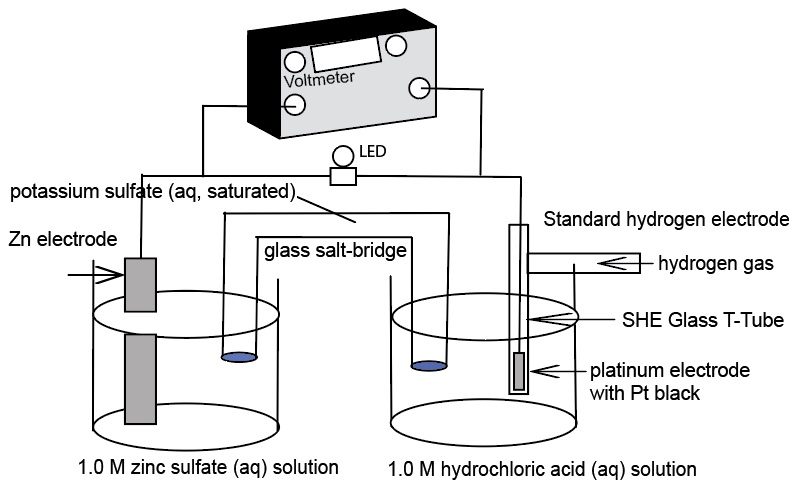
Diagrams of the two electrochemical cells incorporating a standard hydrogen electrode as one of the half-cells: Copper/Cu2+ half-cell and Zn/Zn2+ half-cell.
 |  |
The Voltaic Cell computer simulation is used to show the E°cell generated in order to determine the standard reduction potentials of two half-reactions:
Zn2++ 2e- -> Zn E° = -0.76 V and Cu2++ 2e- -> Cu E° = +0.34 V.
Also, the simulation has animations of what occurs at the surface of the SHE platinum electrode when it serves as an anode and when it serves as a cathode.
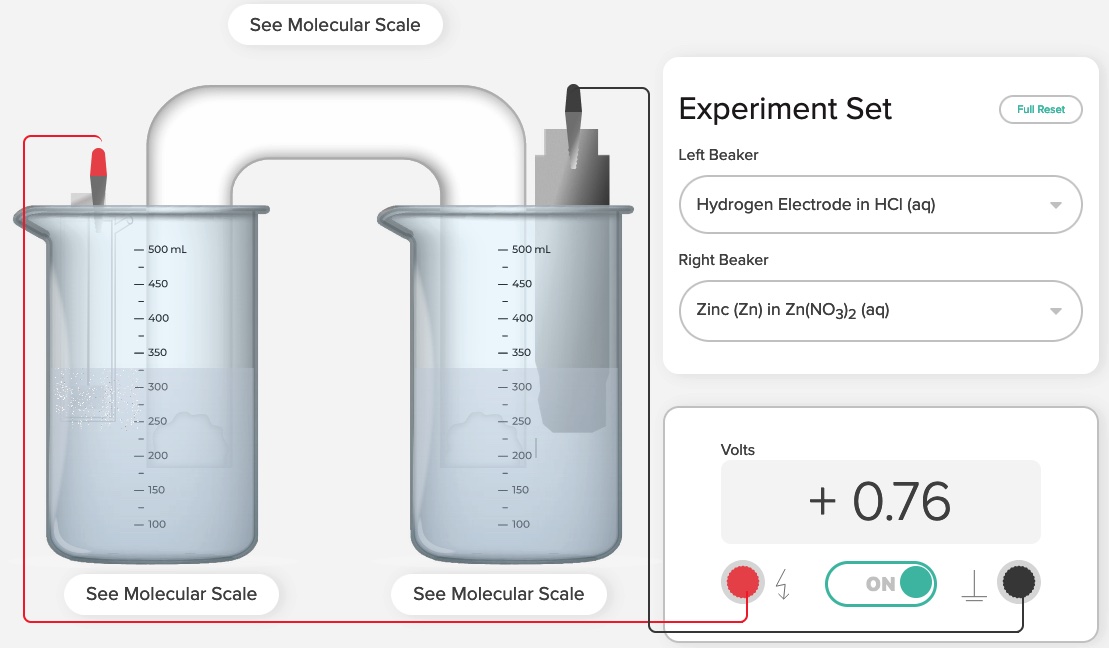 | 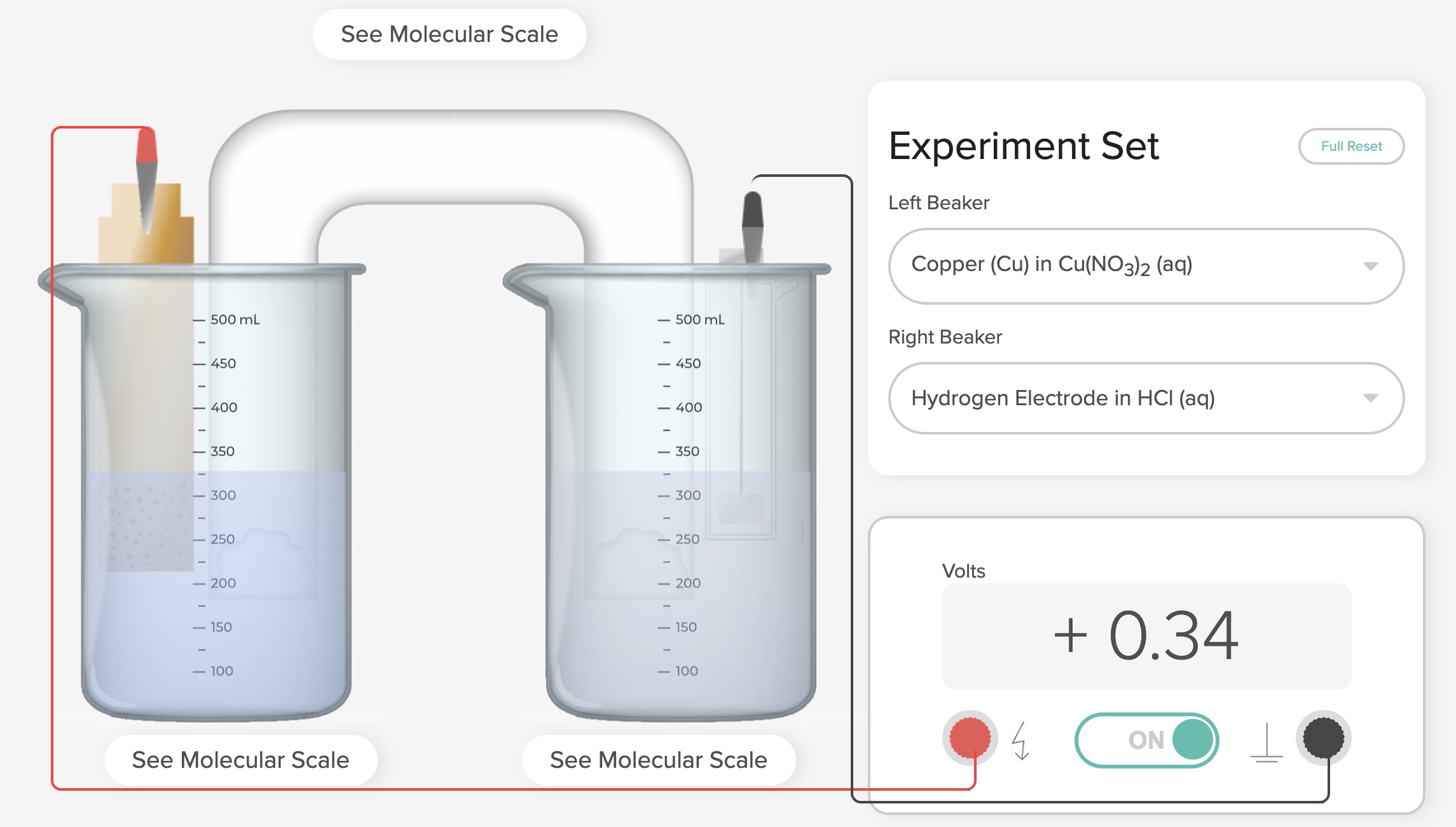 |
Connecting the more active half-cell to the "ground" terminal of the voltmeter (using black wires) ensures a positive voltage - a spontaneous reaction.
ACS AACT Voltaic Cells 1 Greenbowe, T.J.; Gelder, J.I., Boyd, A, Wixon, M. (2020). Galvanic/Voltaic Cells 1. American Chemical Society, American Association of Chemistry Teachers, Washington, D.C. https://teachchemistry.org/classroom-resources/voltaic-cells
Web page author: T. Greenbowe, University of Oregon. This page is under construction.
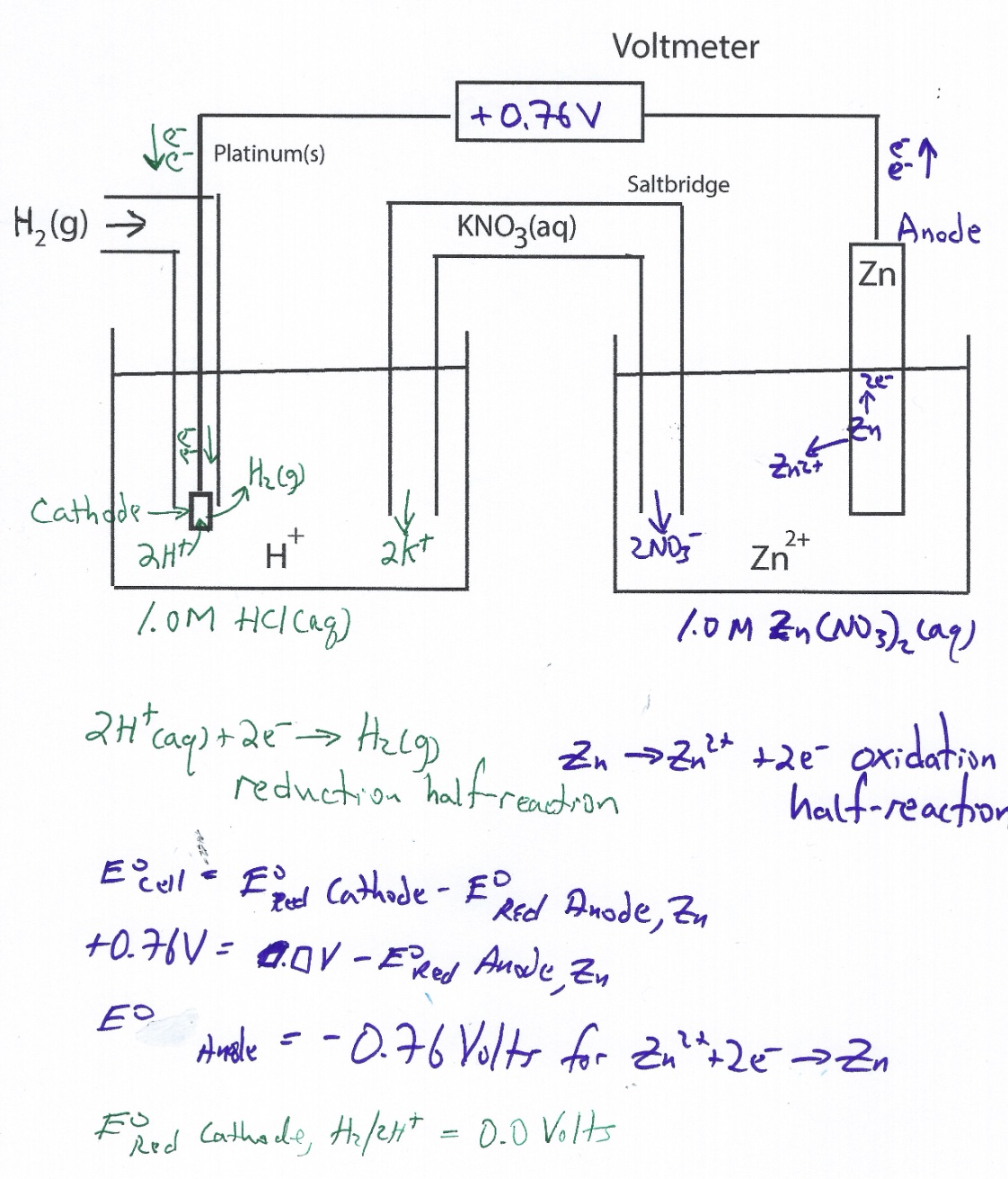
When showing the real SHE Zn cell, Zn|Zn2+|| SHE, make the connections to the voltmeter such that the cell generates a positive voltage - the cell reaction needs to be spontaneous and this calls for a positive E°cell value. Zinc a more active metal compared to the SHE serves as the anode. Oxidation occurs at the anode.
Zn -> Zn2+ + 2e-
The SHE half-cell serves as the cathode in this electrochemical cell. By definition, the SHE reduction potential is zero volts.
2H+(aq) + 2e- -> H2(g, 1 atm) E° = 0.0 V
The goal is to determine the value of E° of the zinc half-cell reduction reaction.
Zn2+ + 2e- -> Zn E° = ?
Standard reduction potentials are all written as reduction half-reactions.
Using the potential difference approach, allows for the determination of E° of the zinc half-cell reduction reaction.
E°cell = E°reduction (Cathode) - E°reduction (Anode) Using this formula, all E° values are reduction potentials.
+0.76 V = 0.0 V - E°reduction (Anode)
E°reduction (Anode) = -0.76 V
Zn2++ 2e- -> Zn E° = -0.76 V
An animation at the particle level when the SHE serves as a cathode and zinc serves as the anode.
2H+(aq) + 2e- --> H2(g) (an oxidation half-reaction, occurs at the anode)
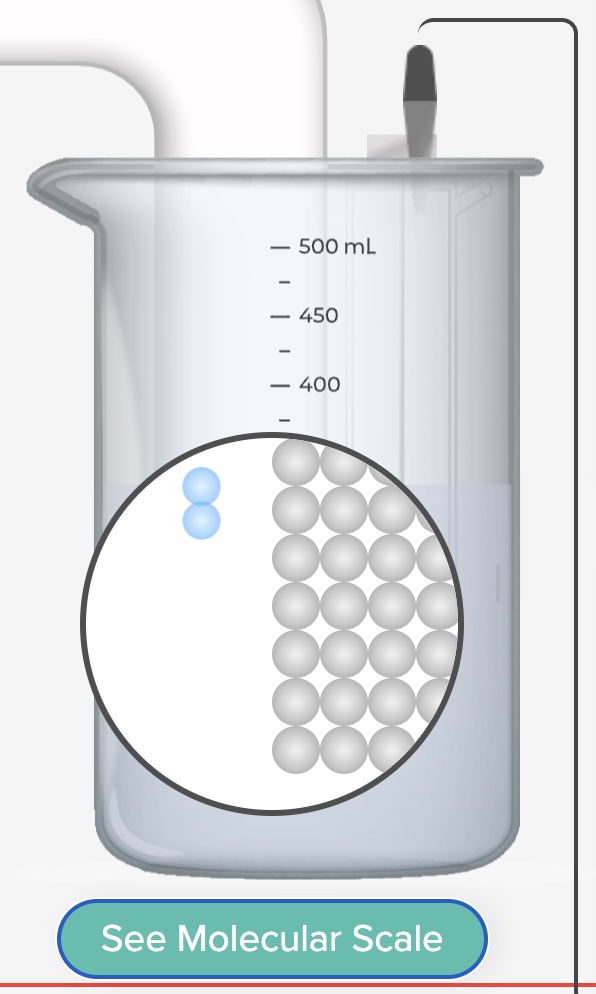
2H+(aq) + 2e- --> H2(g) (a reduction half-reaction, occurs at the cathode)
Zn -> Zn2+ + 2e- (an oxidation half-reaction, occurs at the anode)
[animation to be posted here]
For the SHE || Cu2+ | Cu where the SHE is more active compared to copper. The SHE serves as the anode.
For the SHE || Cu2+ | Cu
An animation at the particle level when the SHE serves as a anode and copper serves as the cathode.
H2(g) --> 2H+(aq) + 2e- (an oxidation half-reaction, occurs at the anode)
ACS AACT https://teachchemistry.org/classroom-resources/voltaic-cells Greenbowe, T.J.; Gelder, J.I., Boyd, A, Wixon, M. (2020). Galvanic/Voltaic Cells 1. American Chemical Society, American Association of Chemistry Teachers, Washington, D.C. https://teachchemistry.org/classroom-resources/voltaic-cells
[Copper half-cell animation to be posted here]
Cu2++ 2e- -> Cu E° = +0.34 V for SHE||Cu2+|Cu electrochemical cells. The copper electrode, being less active compared to the SHE, is the site of reduction (cathode)
E°cell = E°reduction (Cathode) - E° reduction (Anode)
+0.34 V = E°reduction (Cathode) - 0.0 V
+0.34 V = E°reduction (Cathode)
Construct a real Zn-Cu cell using the two half-cells and construct a Zn-Cu cell using the AACT Voltaic Cell computer simulation.
After the individual half-cell emfs are determined, combine the half-cells to give the E° cell for
Zn + Cu2+(aq) -> Zn2+(aq) + Cu E° = ?
Cu2++ 2e- -> Cu E° = +0.34 V
2H+(aq) +2e- -> H2(g, 1 atm) E° = 0.0 V
Zn2++ 2e- -> Zn E° = -0.76 V
E°cell = E°reduction (Cathode) - E° reduction (Anode)
E°cell = +0.34 V - (-0.76 V) = +1.10 V
Curriculum Notes
A Standard Hydrogen Electrode is used to show the E°cell generated: SHE||Cu2+|Cu and Zn|Zn2+||SHE. The effectiveness of this demonstration can be increased by showing computer animations representing what occurs at the anode and cathode of each cell at the particle or atom level.
Computer animations for the SHE electrode (drafts):
Zn|Zn2+||SHE electrochemical cells oxidation half-reaction at the zinc electrode https://vimeo.com/220550690 Zn -> Zn2++ 2e-
reduction half-reaction at SHE https://vimeo.com/220550539 2H+(aq) +2e- -> H2(g, 1 atm) E° = 0.0 V
SHE||Cu2+|Cu electrochemical cell oxidation half-reaction at SHE https://vimeo.com/220550498 H2(g, 1 atm) -> 2H+(aq) +2e- E° = 0.0 V
reduction half-reaction at the copper electrode https://vimeo.com/220550267 Cu2++ 2e- -> Cu E° = +0.34 V
including the computer simulations & animations provides an excellent opportunity for students to connect the macroscopic, microscopic (particle/atom) and symbolic levels of representation on Johnstone's Triangle.
Zinc - standard hydrogen electrode electrochemical cell computer animation
https://www.youtube.com/watch?v=CQ7gYg64Msc
Active Learning A POGIl Activity can accompany this demonstration to involve students in active learning. Rather than lecturing to students, have the students use the SHE metal electrode block diagrams to take notes on what they observe from the demonstration and from the computer animation of what occurs at the surface of the SHE electrode and the metal electrode for the ZnShe cell and the SHECu cell. Have students identify where oxidation and reduction occurs and have the students write the half-reactions.
Example of student work
Clicker questions are available to support and enhance the demonstration and the computer simulations & animations.
Lecture presentation and description of a Standard Hydrogen Electrode - video by KEGS. Narration and CC.

Learning Objectives
1. Describe and identify the components of a Standard Hydrogen Electrode.
2. Write the half-reactions that occurs in a Standard Hydrogen Electrode when it is acting as a cathode. Write the half-reactions that occurs in a Standard Hydrogen Electrode when it is acting as an anode.
3. Explain how a Standard Hydrogen Electrode is used as a reference electrode (E° = 0.0 V) to measure E° half-cell values.
4. Show how a Standard Hydrogen Electrode can be used to rank half-cell reactions and determine an emf series.
5. Show how E° half-cells are combined to give the E° cell.
6. Show that ∆E° is the difference in emf between two half-cell potentials. Use a number line to emphasize this point.
References
Abraham, M.; Gelder, J.; Greenbowe, T. (2007). During Class Inventions and Computer Lab Activities for First and Second Semester General Chemistry. Hayden-McNeil: Plymouth, MI.
Greenbowe, T.J. An Interactive Multimedia Software Program for Exploring Electrochemical Cells. J. Chem. Educ., 1994, 71 (7), p 555.
Greenbowe, T.J.; Gelder, J.I., Boyd, A, Wixon, M. (2020). Galvanic/Voltaic Cells 1. American Chemical Society, American Association of Chemistry Teachers,
Sanger, M.J. and Greenbowe, T.J. (1997). “Student Misconceptions in Electrochemistry: Current Flow in Electrolyte Solutions and the Salt Bridge.” Journal of Chemical Education, 74(7), 819-823.
Sanger, M. J. and Greenbowe, T.J. (1997). “Common Student Misconceptions in Electrochemistry: Galvanic, Electrolytic, and Concentration Cells.” Journal of Research in Science Teaching, 34(4), 377-398.
Sanger, M.J. and Greenbowe, T.J. (1999). “An Analysis of College of Chemistry Textbooks as Sources of Misconception and Errors in Electrochemistry.” Journal of Chemical Education, 76(6), 853-860.
