Electrochemistry
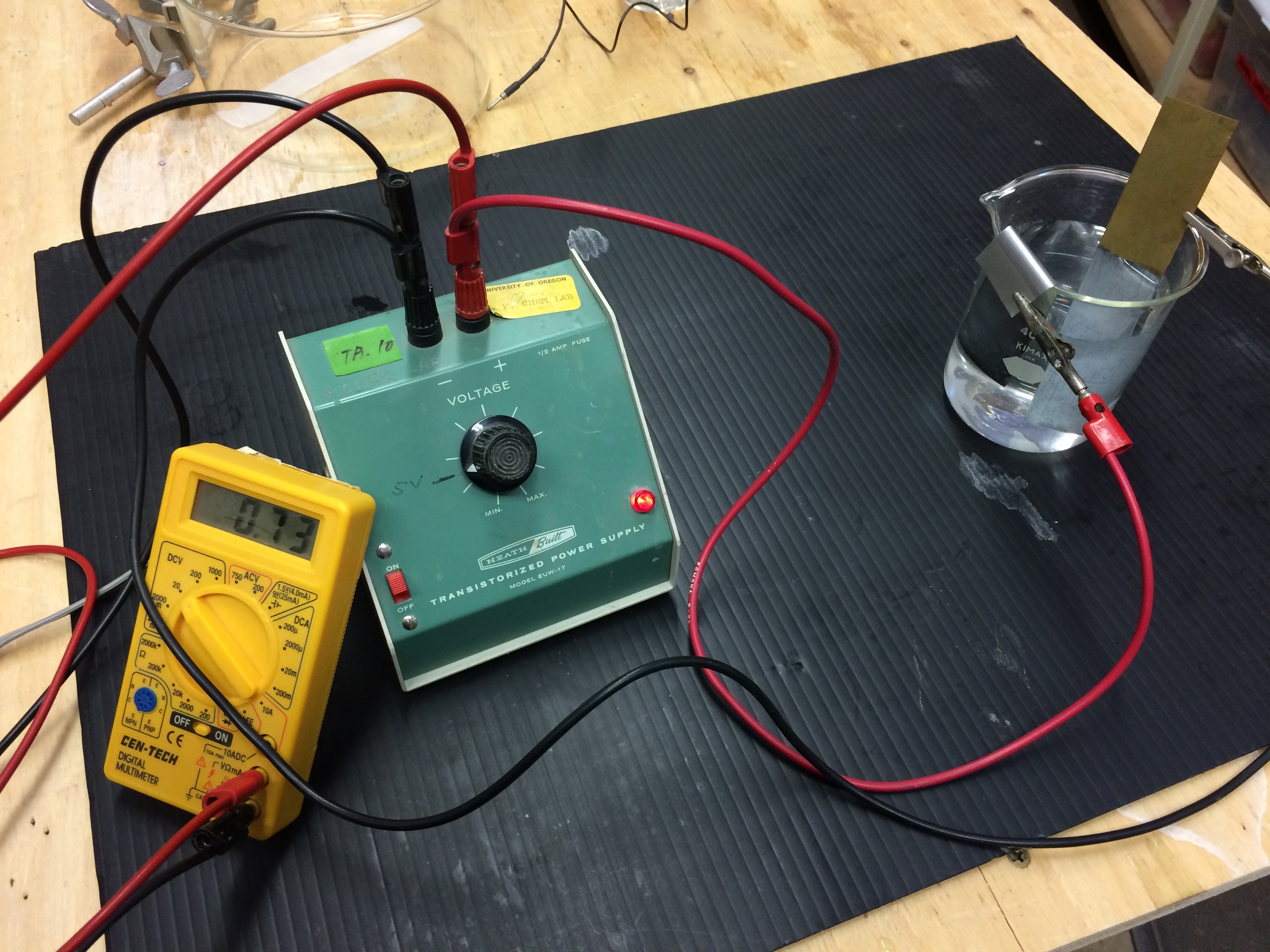
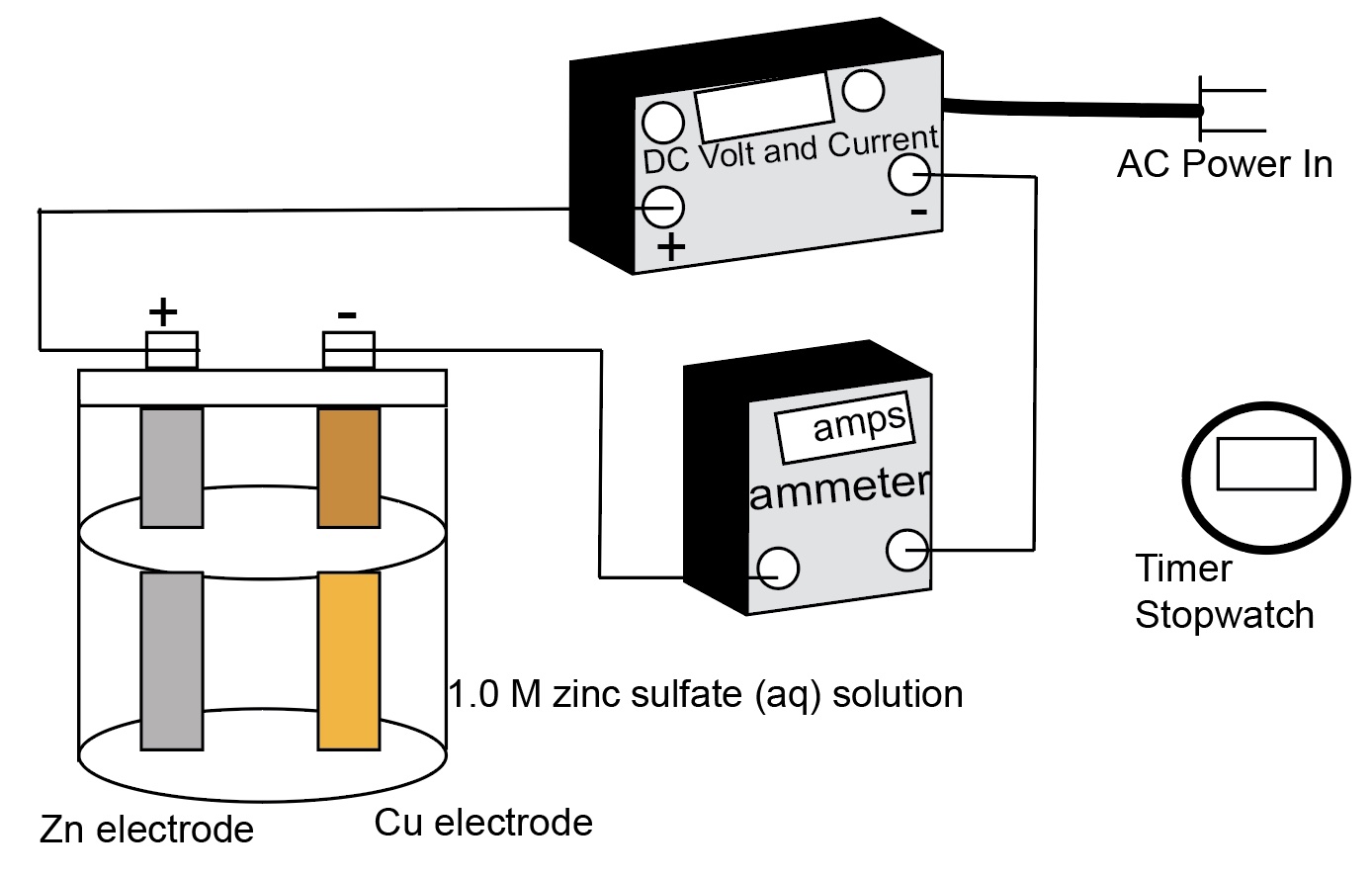
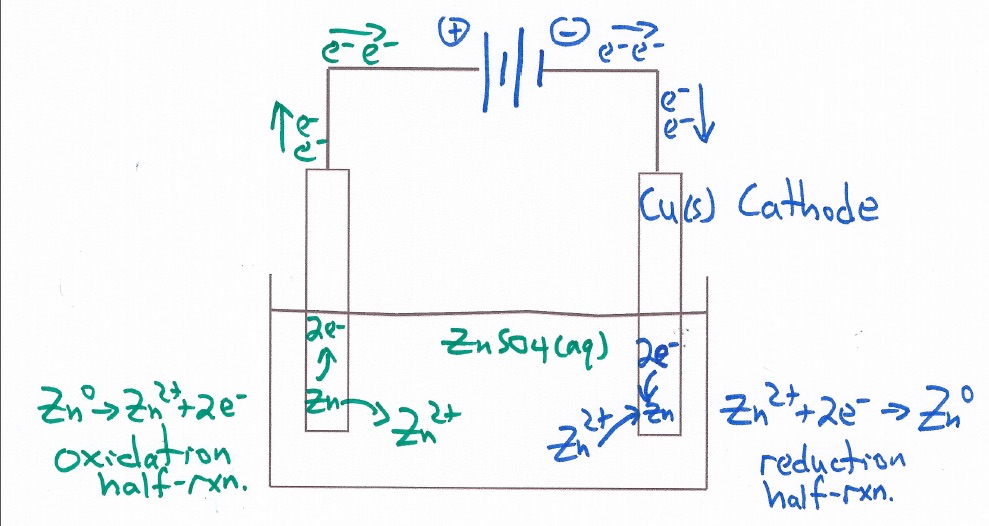
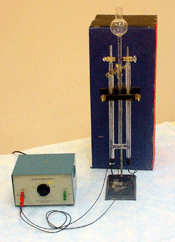
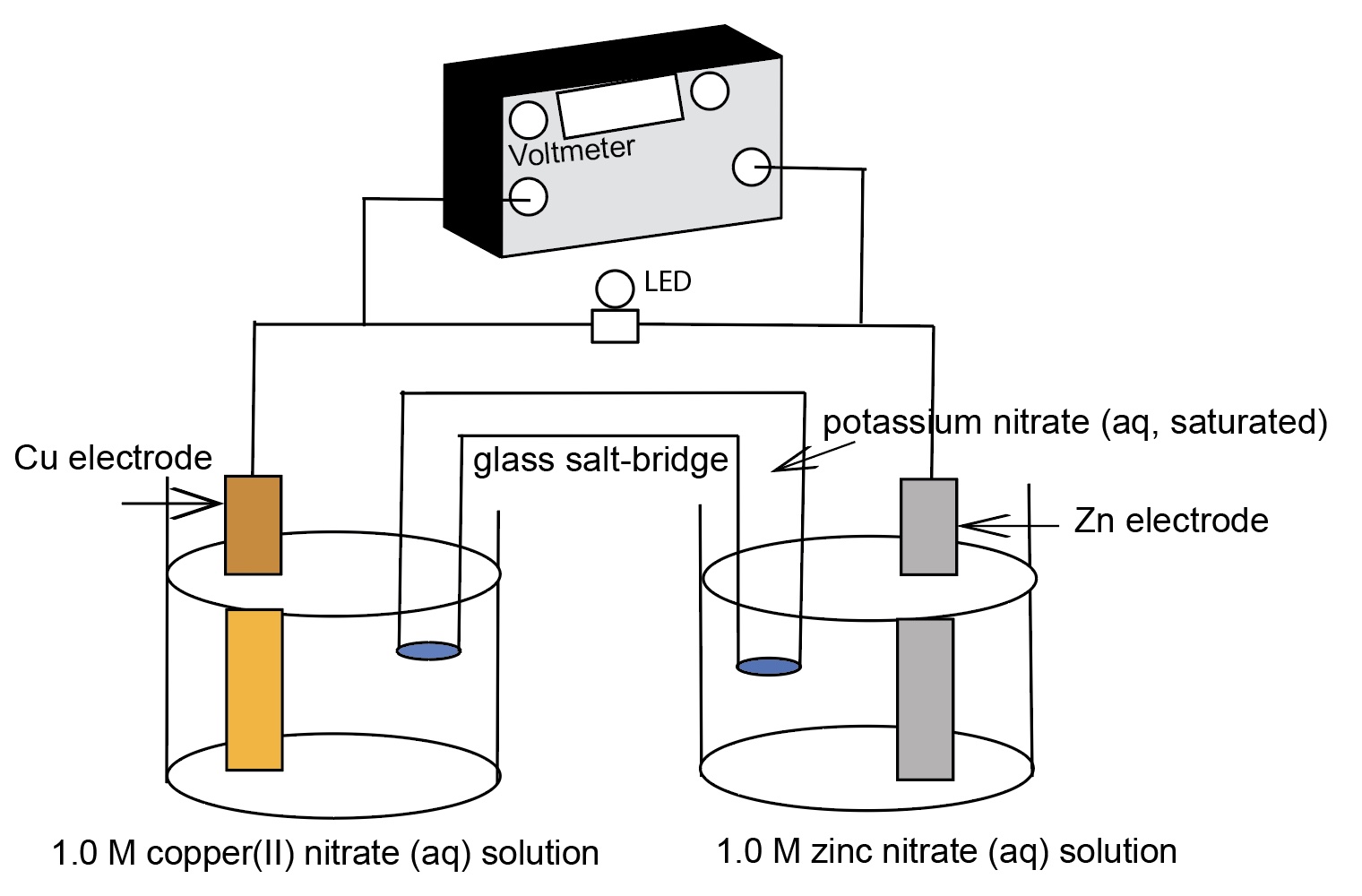
Electrochemcial Cell Demonstration Voltaic Cell: Zinc/Copper
Demonstration of generating electricity from a chemical oxidation-reduction reaction. The process involves moving electrons.
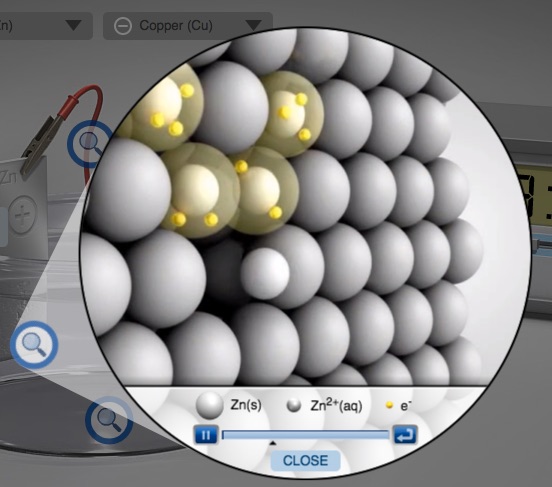
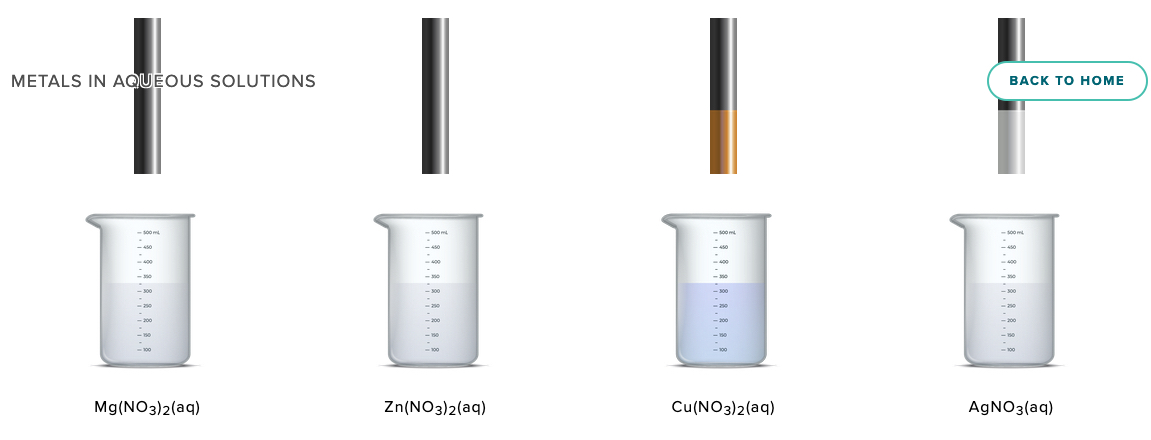
Activity Series of Metals Computer Simulation Metals in Aqueous Solution AACT
Select various metals to test in aqueous M2+ solutions.
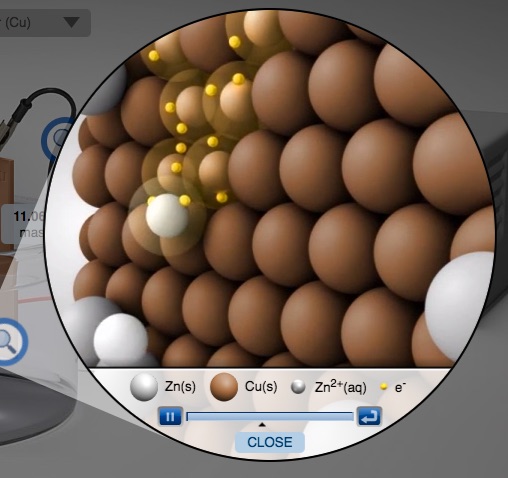
Electrolysis Computer Simulation New HTML5 Version Plate Cu on Cu
Electrolysis computer simulation of a various metal-metal electrolytic cells. Choose metal electrodes, electrolyte, current and time.
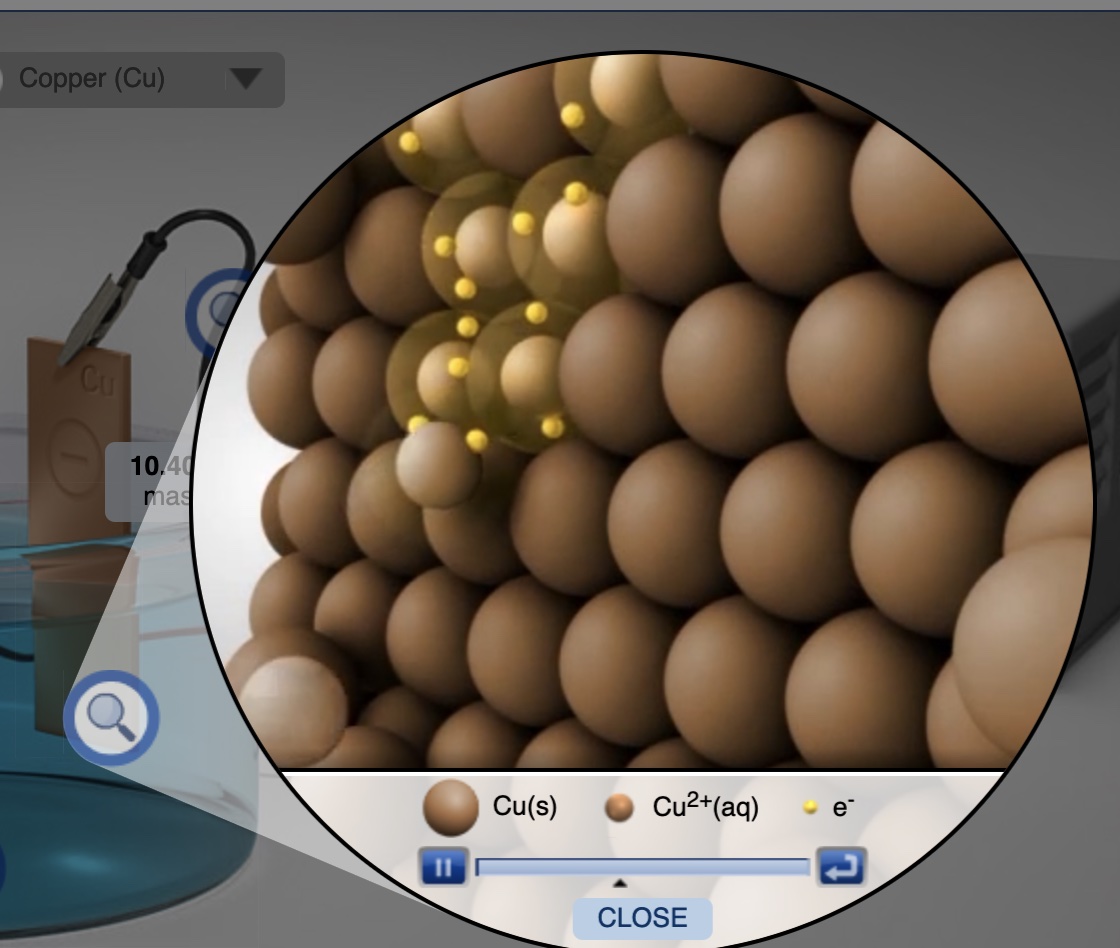
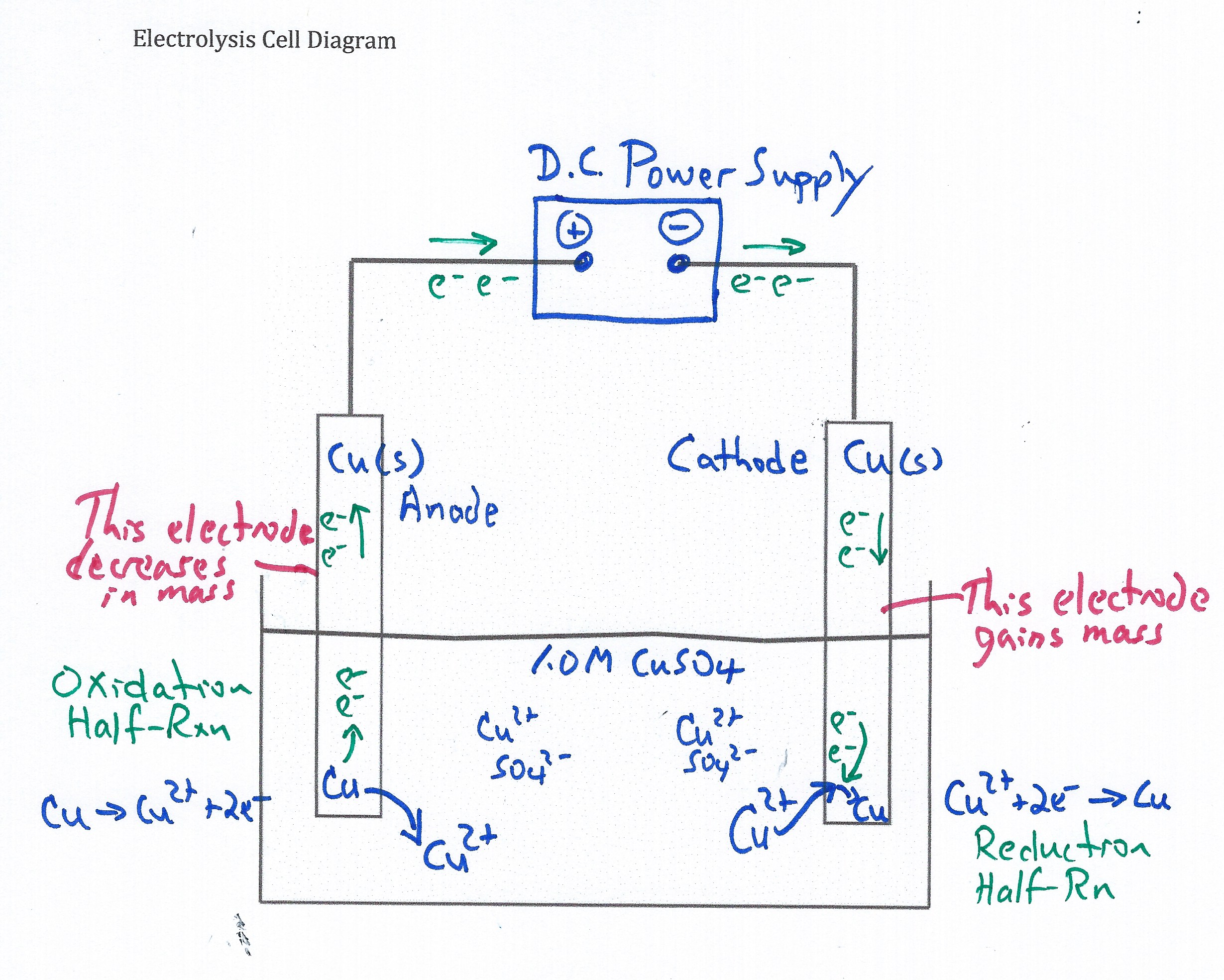
Electrolysis Cell: Plating Copper on Copper Demonstration - Real and Computer Simulation
A copper-copper electrolytic cell is built comprised of two copper electrodes in 1.0 M CuSO4(aq) connected to a DC voltage-current generator. The initial mass of both electrodes are measured. Students observe two shiny copper metal electrodes at the start of the demonstration. An ammeter measures the applied current. When current is applied to the electrolysis cell copper(II) ions are reduced to copper atoms at the cathode and copper atoms are oxidized to copper(II) ions at the anode. Within two minutes students observe discoloration on both of the copper electrodes. The time to plate copper metal on the cathode is recorded along with the current (amps). At the end of the electroysis experiment, the mass of both electrodes are measured. When this demonstration is combined with the Electrolysis Simulation students easily see and understand 1) the function of the DC Power Supply (forces electrons into the cathode and pulls electrons out of the anode); 2) what half-reactions occur at each electrode; 3) the relationship between current, time, ion charge and moles of metal plated which leads to an understanding of Faraday's Law of Electrolysis; and 4) the differences between electrolytic cells and galvanic (voltaic) cells.